当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
cis-β-Ruthenium Complexes with Sterically Bulky Salen Ligands: Enantioselective Intermolecular Carbene Insertion into Si–H Bonds and X-ray Crystal Structure of cis-β-[RuII(salen)(CO)(CPh2)] Complex
Organometallics ( IF 2.5 ) Pub Date : 2020-07-07 , DOI: 10.1021/acs.organomet.0c00268
Chi Lun Lee 1 , Daqing Chen 1, 2 , Xiao-Yong Chang 1 , Zhou Tang 1 , Chi-Ming Che 1, 3
Organometallics ( IF 2.5 ) Pub Date : 2020-07-07 , DOI: 10.1021/acs.organomet.0c00268
Chi Lun Lee 1 , Daqing Chen 1, 2 , Xiao-Yong Chang 1 , Zhou Tang 1 , Chi-Ming Che 1, 3
Affiliation
|
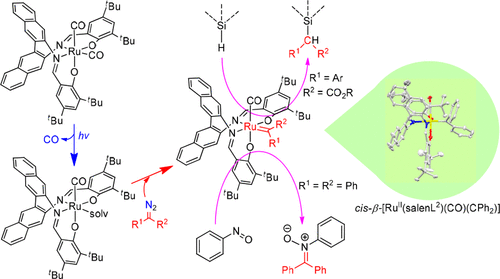
The synthesis, spectroscopy, crystal structure, and electrochemical behavior of cis-β-RuII(CO)2, cis-β-RuII(CO)(H2O), and cis-β-RuII(CO)(carbene) complexes supported by sterically bulky salen ligands are described, along with the catalytic activity of chiral cis-β-[RuII(salen)(CO)2] complexes toward enantioselective carbene Si–H insertion using N2C(Ar)CO2R as the carbene source under light irradiation with product yields up to 96% and ee up to 84%. The cis-β-RuII(CO)(H2O) complex was isolated by using a binaphthyl salen ligand bearing bulky CPh3 substituents with the H2O molecule coordinated at the site trans to an Nimine atom of the salen ligand. Light irradiation of a cis-β-RuII(CO)2 complex, supported by a binaphthyl salen ligand with tBu substituents, followed by treatment with N2CAr2 (Ar = Ph, p-ClC6H4) gave cis-β-[RuII(salen)(CO)(CAr2)], which features a coordinated carbene ligand trans to a salen Nimine atom and undergoes carbene transfer to nitrosobenzene to give the corresponding nitrone product. These findings and related HR-ESI-MS studies provide evidence for the involvement of salen-supported cis-β-RuII(CO)(C(Ar)CO2R) active species in the intermolecular carbene Si–H insertion reaction catalyzed by cis-β-[RuII(salen)(CO)2] complexes.
中文翻译:

带有大体积Salen配体的顺式-β-钌配合物:对映选择性分子间碳原子插入Si-H键和X-射线晶体结构的顺式-β- [Ru II(salen)(CO)(CPh 2)]配合物
合成,光谱,晶体结构和电化学行为顺式β-钌II(CO)2,顺式β-钌II(CO)(H 2 O),和顺式β-钌II(CO)(卡宾)描述了由体积庞大的Salen配体支持的配合物,以及使用N 2 C(Ar)CO 2的手性顺式β- [Ru II(salen)(CO)2 ]配合物对对映选择性卡宾Si–H插入的催化活性。在光照射下,R作为卡宾源,产品收率高达96%,ee高达84%。该顺β-茹II(CO)(H 2 O)络合物是通过使用带有庞大CPh 3取代基的双萘基Salen配体进行分离的,其中H 2 O分子在与SALEN配体的N亚胺原子反位的位点配位。在具有t Bu取代基的双萘基Salen配体的支持下,对顺-β- Ru II(CO)2络合物进行光照射,然后用N 2 CAr 2(Ar = Ph,p -ClC 6 H 4)处理,得到顺- β- [Ru II(salen)(CO)(CAr 2)],其特征在于将配位的卡宾配体反式转化为Salen N亚胺原子,并将卡宾转移至亚硝基苯,得到相应的硝酮产物。这些发现和相关的HR-ESI-MS研究提供了萨伦支持的参与证据顺式β-钌II(CO)(C(Ar)的CO 2 R)在分子间卡宾活性种的Si-H插入反应催化顺式-β- [Ru II(salen)(CO)2 ]配合物。
更新日期:2020-07-27
中文翻译:

带有大体积Salen配体的顺式-β-钌配合物:对映选择性分子间碳原子插入Si-H键和X-射线晶体结构的顺式-β- [Ru II(salen)(CO)(CPh 2)]配合物
合成,光谱,晶体结构和电化学行为顺式β-钌II(CO)2,顺式β-钌II(CO)(H 2 O),和顺式β-钌II(CO)(卡宾)描述了由体积庞大的Salen配体支持的配合物,以及使用N 2 C(Ar)CO 2的手性顺式β- [Ru II(salen)(CO)2 ]配合物对对映选择性卡宾Si–H插入的催化活性。在光照射下,R作为卡宾源,产品收率高达96%,ee高达84%。该顺β-茹II(CO)(H 2 O)络合物是通过使用带有庞大CPh 3取代基的双萘基Salen配体进行分离的,其中H 2 O分子在与SALEN配体的N亚胺原子反位的位点配位。在具有t Bu取代基的双萘基Salen配体的支持下,对顺-β- Ru II(CO)2络合物进行光照射,然后用N 2 CAr 2(Ar = Ph,p -ClC 6 H 4)处理,得到顺- β- [Ru II(salen)(CO)(CAr 2)],其特征在于将配位的卡宾配体反式转化为Salen N亚胺原子,并将卡宾转移至亚硝基苯,得到相应的硝酮产物。这些发现和相关的HR-ESI-MS研究提供了萨伦支持的参与证据顺式β-钌II(CO)(C(Ar)的CO 2 R)在分子间卡宾活性种的Si-H插入反应催化顺式-β- [Ru II(salen)(CO)2 ]配合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号