当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heterogeneous Catalytic Oxidation of Furfural with Hydrogen Peroxide over Sulfated Zirconia
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-07-07 , DOI: 10.1021/acs.iecr.0c02566 Dmitry Yu. Murzin 1 , Emilie Bertrand 1 , Pasi Tolvanen 1 , Sergey Devyatkov 1 , Jani Rahkila 2 , Kari Eränen 1 , Johan Wärnå 1 , Tapio Salmi 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-07-07 , DOI: 10.1021/acs.iecr.0c02566 Dmitry Yu. Murzin 1 , Emilie Bertrand 1 , Pasi Tolvanen 1 , Sergey Devyatkov 1 , Jani Rahkila 2 , Kari Eränen 1 , Johan Wärnå 1 , Tapio Salmi 1
Affiliation
|
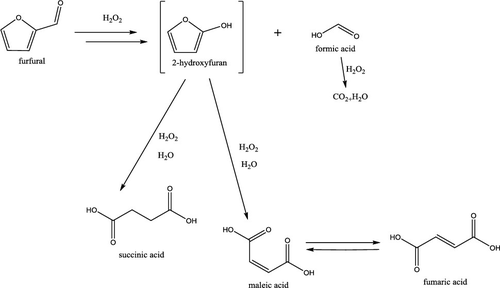
Furfural oxidation with hydrogen peroxide was performed using sulfated zirconia as an acid catalyst within the temperature range of 40–90 °C. The catalytic behavior of sulfated zirconia due to its acidic character was different from a previously studied fibrous polymer-supported sulfonic acid catalyst Smopex-101. While maleic and succinic acids along with 2(5-H)-furanone and formic acid were formed, selectivity to succinic acid was lower. Moreover, significant amounts of 5-hydroxy-2(5-H)-furanone were generated as confirmed by nuclear magnetic resonance (NMR). In parallel to furfural oxidation giving products with four carbon atoms and formic acid, also furoic acid was formed and oxidized further. The reaction network proposed previously was modified and extended to account for these observations. In particular, the oxidation of 2(5-H)-furanone to maleic acid, considered to be a plausible step over Smopex-101, turned out to be very slow in the presence of sulfated zirconia. The kinetic model developed on the basis of the reaction network was able to describe the concentration profiles of the reactants, intermediates, and products as a function of reaction time at different temperatures including decomposition of hydrogen peroxide.
中文翻译:

硫酸锆对过氧化氢催化糠醛的非均相催化氧化
在40-90°C的温度范围内,使用硫酸化氧化锆作为酸催化剂,用过氧化氢进行糠醛氧化。硫酸化氧化锆由于其酸性特征而具有的催化行为不同于先前研究的纤维状聚合物负载的磺酸催化剂Smopex-101。虽然形成了马来酸和琥珀酸以及2(5-H)-呋喃酮和甲酸,但对琥珀酸的选择性较低。此外,如核磁共振(NMR)所证实,产生了大量的5-羟基-2(5-H)-呋喃酮。与糠醛氧化平行,得到具有四个碳原子和甲酸的产物,还形成了糠酸并进一步氧化。先前提出的反应网络已进行了修改和扩展,以解决这些问题。尤其是,2(5-H)-呋喃酮氧化成顺丁烯二酸的步骤被认为是在Smopex-101上进行的一个可行步骤,但在存在硫酸化氧化锆的情况下,氧化过程非常缓慢。在反应网络的基础上建立的动力学模型能够描述反应物,中间体和产物的浓度分布随反应时间在不同温度(包括过氧化氢分解)的变化。
更新日期:2020-07-29
中文翻译:

硫酸锆对过氧化氢催化糠醛的非均相催化氧化
在40-90°C的温度范围内,使用硫酸化氧化锆作为酸催化剂,用过氧化氢进行糠醛氧化。硫酸化氧化锆由于其酸性特征而具有的催化行为不同于先前研究的纤维状聚合物负载的磺酸催化剂Smopex-101。虽然形成了马来酸和琥珀酸以及2(5-H)-呋喃酮和甲酸,但对琥珀酸的选择性较低。此外,如核磁共振(NMR)所证实,产生了大量的5-羟基-2(5-H)-呋喃酮。与糠醛氧化平行,得到具有四个碳原子和甲酸的产物,还形成了糠酸并进一步氧化。先前提出的反应网络已进行了修改和扩展,以解决这些问题。尤其是,2(5-H)-呋喃酮氧化成顺丁烯二酸的步骤被认为是在Smopex-101上进行的一个可行步骤,但在存在硫酸化氧化锆的情况下,氧化过程非常缓慢。在反应网络的基础上建立的动力学模型能够描述反应物,中间体和产物的浓度分布随反应时间在不同温度(包括过氧化氢分解)的变化。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号