当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
2D MOFs with Ni(II), Cu(II), and Co(II) as Efficient Oxygen Evolution Electrocatalysts: Rationalization of Catalytic Performance vs Structure of the MOFs and Potential of the Redox Couples.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-07-07 , DOI: 10.1021/acsami.0c07268 Anindita Goswami 1 , Debanjali Ghosh 2 , Vladimir V Chernyshev 3 , Avishek Dey 1 , Debabrata Pradhan 2 , Kumar Biradha 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-07-07 , DOI: 10.1021/acsami.0c07268 Anindita Goswami 1 , Debanjali Ghosh 2 , Vladimir V Chernyshev 3 , Avishek Dey 1 , Debabrata Pradhan 2 , Kumar Biradha 1
Affiliation
|
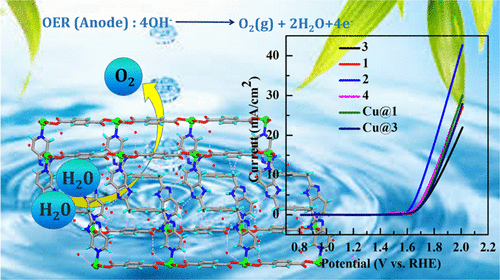
Earth-abundant transition-metal-based metal–organic frameworks (MOFs) are of immense interest for the development of efficient and durable heterogeneous water splitting electrocatalysts. This repot explores the design of two-dimensional (2D) MOFs with redox-active metal centers (Ni(II), Co(II), and Cu(II)) containing two types of electron-rich linkers such as bis(5-azabenzimidazole), linear L1 and angular L2, and aromatic dicarboxylates. The electron-rich linkers are considered to stabilize the higher oxidation state of the redox-active metal centers in the course of the electrocatalytic oxygen evolution reaction (OER) process. The 2D MOFs of L1 and L2 with Co(II) (1 and 3) and Ni(II) (2 and 4) have been produced via the conventional hydrothermal synthesis, while the MOFs of Cu(II) (Cu@1 and Cu@3) are obtained by the postsynthetic transmetallation reaction of MOFs 1 and 3. The electrocatalytic OER activities of the six MOFs have been studied to explore the influence of the redox potential of the transition-metal quasi-reversible couples and the coordination environment around the redox-active metal centers in the electrocatalytic activity. The lowest overpotential of 370 mV exhibited by MOF 2 with the highest current density and TOF value indicates the importance of the presence of coordinated water molecules and the lowest redox potential value of the most favorable quasi-reversible couple Ni+2/Ni+3. These catalysts exhibit a remarkable stability up to 1000 OER cycles. These studies pave the way for the design of MOF materials toward the development of a promising heterogeneous OER electrocatalyst.
中文翻译:

以Ni(II),Cu(II)和Co(II)作为高效的析氧电催化剂的2D MOF:催化性能与MOF的结构和氧化还原电对势的合理化。
富含地球的过渡金属基金属有机框架(MOF)对于开发高效,耐用的非均相水分解电催化剂非常重要。此报告探讨具有氧化还原活性金属中心(Ni(II),Co(II)和Cu(II))的二维(2D)MOF的设计,该中心包含两种类型的富电子连接基,例如bis(5-氮杂苯并咪唑),线性L 1和角L 2以及芳族二羧酸盐。富电子连接子被认为可以在电催化氧放出反应(OER)过程中稳定氧化还原活性金属中心的较高氧化态。L 1和L 2与Co(II)(1的2D MOF和3)和Ni(II)(2和4)是通过常规水热合成制备的,而Cu(II)的MOF(Cu @ 1和Cu @ 3)是通过MOFs 1和MOF的后合成金属化反应获得的。3。研究了六个MOFs的电催化OER活性,以探讨过渡金属准可逆对的氧化还原电势和氧化还原活性金属中心周围的配位环境对电催化活性的影响。MOF 2展示的最低过电势为370 mV电流密度和TOF值最高的离子表示存在配位水分子的重要性,而最有利的准可逆偶合物Ni + 2 / Ni +3的氧化还原电位值最低。这些催化剂在高达1000 OER的循环中表现出出色的稳定性。这些研究为MOF材料的设计铺平了道路,以开发有前途的多相OER电催化剂。
更新日期:2020-07-07
中文翻译:

以Ni(II),Cu(II)和Co(II)作为高效的析氧电催化剂的2D MOF:催化性能与MOF的结构和氧化还原电对势的合理化。
富含地球的过渡金属基金属有机框架(MOF)对于开发高效,耐用的非均相水分解电催化剂非常重要。此报告探讨具有氧化还原活性金属中心(Ni(II),Co(II)和Cu(II))的二维(2D)MOF的设计,该中心包含两种类型的富电子连接基,例如bis(5-氮杂苯并咪唑),线性L 1和角L 2以及芳族二羧酸盐。富电子连接子被认为可以在电催化氧放出反应(OER)过程中稳定氧化还原活性金属中心的较高氧化态。L 1和L 2与Co(II)(1的2D MOF和3)和Ni(II)(2和4)是通过常规水热合成制备的,而Cu(II)的MOF(Cu @ 1和Cu @ 3)是通过MOFs 1和MOF的后合成金属化反应获得的。3。研究了六个MOFs的电催化OER活性,以探讨过渡金属准可逆对的氧化还原电势和氧化还原活性金属中心周围的配位环境对电催化活性的影响。MOF 2展示的最低过电势为370 mV电流密度和TOF值最高的离子表示存在配位水分子的重要性,而最有利的准可逆偶合物Ni + 2 / Ni +3的氧化还原电位值最低。这些催化剂在高达1000 OER的循环中表现出出色的稳定性。这些研究为MOF材料的设计铺平了道路,以开发有前途的多相OER电催化剂。







































 京公网安备 11010802027423号
京公网安备 11010802027423号