Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-07-06 , DOI: 10.1016/j.bioorg.2020.104074 Qiong Wu 1 , Yue Song 2 , Ruotong Liu 3 , Rui Wang 2 , Wenjie Mei 4 , Weiming Chen 3 , Huanglan Yang 3 , Xicheng Wang 2
|
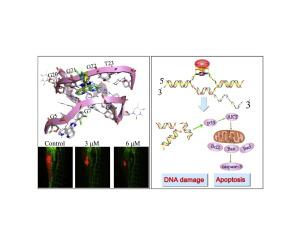
Phenanthroimidazole derivatives containing phenanthroline and imidazole heterocyclic aromatic rings are effective agents to inhibit tumor cell growth. Herein, halogen element-modified imidazo[4,5f][1,10]phenanthroline derivatives 1–6 (1, 4-fluorophenyl; 2, 4-chlorophenyl; 3, 4-bromobenyl; 4, 2,3-dichlorophenyl; 5, 3,4-dichlorophenyl; and 6, 2,4-dichlorophenyl) were synthesized, and their antitumor activities were investigated. All of the compounds, especially 4, exhibited an excellent inhibitory effect against nasopharyngeal carcinoma CNE-1 cells. This effect was better than that of doxorubicin. Compound 4 also markedly blocked the proliferation of the CNE-1 cells in a zebrafish xenograft model. The antitumor mechanisms might be attributed to apoptosis induction, which triggered ROS-mediated DNA damage and generated mitochondrial dysfunction by stabilizing c-myc G-quadruplex DNA structure. Results indicated that phenanthroimidazole derivatives could act as promising anticancer agents.
中文翻译:

菲并咪唑衍生物作为有前途的c-myc G-四链体DNA稳定剂的合成,对接研究和抗肿瘤活性。
含有菲咯啉和咪唑杂环芳环的菲并咪唑衍生物是抑制肿瘤细胞生长的有效药物。在本文中,卤族元素改性的咪唑并[4,5 ˚F ] [1,10]菲咯啉衍生物1-6(1,4-氟苯基; 2,4-氯苯基; 3,4- bromobenyl; 4,2,3-二氯苯基; 5,3,4-二氯苯基;和6,2,4-二氯苯基)的合成,和它们的抗肿瘤活性进行了研究。所有化合物,特别是4种化合物,对鼻咽癌CNE-1细胞均表现出优异的抑制作用。该作用优于阿霉素。复合图4还显着阻断了斑马鱼异种移植模型中CNE-1细胞的增殖。该抗肿瘤机制可能归因于细胞凋亡的诱导,其通过稳定c-myc G-四链体DNA结构引发ROS介导的DNA损伤并产生线粒体功能障碍。结果表明菲并咪唑衍生物可以作为有希望的抗癌药。








































 京公网安备 11010802027423号
京公网安备 11010802027423号