Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-07-06 , DOI: 10.1016/j.bioorg.2020.104073 Milena D Milošević 1 , Aleksandar D Marinković 2 , Predrag Petrović 3 , Anita Klaus 4 , Milica G Nikolić 5 , Nevena Ž Prlainović 2 , Ilija N Cvijetić 6
|
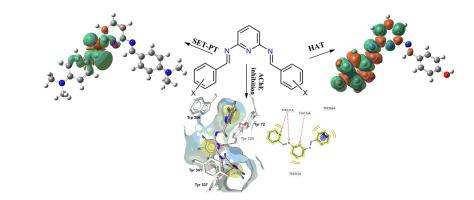
In this study we synthesized a series of sixteen bis(imino)pyridines (BIPs) starting from 2,6-diaminopyridine and various aromatic aldehydes, and evaluated their antioxidant, antibacterial, antifungal and acetylcholinesterase (AChE) inhibitory activity. The chemical structures were elucidated by FTIR, elemental analysis, ESR and HRMS. 1H and 13C NMR spectra couldn’t be acquired due to the formation of stable, carbon-centered radical cations in a solution, as confirmed by ESR spectroscopy and DFT calculations. The in vitro antioxidant potency was evaluated using four assays: free radical scavenging activity (DPPH and ABTS), reducing power and total antioxidant capacity assay. BIPs demonstrated excellent antioxidant properties, and two derivatives proved to be more potent than reference antioxidants (ascorbic acid and Trolox) in all assays. DFT calculations on ωB97XD/6-311++g(d,p) level of theory provided valuable insights into the radical scavenging mechanism of BIPs. For hydroxyl-substituted BIPs, hydrogen atom transfer (HAT) is a predominant mechanism, while the single electron transfer coupled with proton transfer (SET-PT) governs the antioxidant activity of other derivatives. Intramolecular hydrogen bonding (IHB) plays an important role in the mechanism of antioxidant activity as revealed by noncovalent interaction analysis and rotational barrier calculations. The spin density of radical cations is localized on carbon atoms of a pyridine ring, which corroborates with g-factors and multiplicity obtained from ESR analysis. The most potent BIP exhibited moderate inhibitory activity toward AChE (IC50 = 20 ± 4 μM), while molecular docking suggested binding at the peripheral anionic site of AChE with the MMFF94 binding enthalpy of −43.4 kcal/mol. Moderate in vitro antimicrobial activity of BIPs have been determined against several pathogenic bacterial strains: Pseudomonas aeruginosa, Escherichia coli, Enterococcus faecalis, Staphylococcus aureus and clinical isolate of methicillin resistant S. aureus (MRSA). The antifungal activity of BIPs toward Candida albicans was also confirmed. The similarity ensemble approach combined with molecular docking suggested leucyl aminopeptidase as the probable antimicrobial target for the three most potent BIP derivatives.
中文翻译:

双(亚氨基)吡啶作为抗氧化剂,乙酰胆碱酯酶抑制剂和抗菌剂的合成,表征和SAR研究。
在这项研究中,我们从2,6-二氨基吡啶和各种芳香醛开始合成了16种双(亚氨基)吡啶(BIP),并评估了它们的抗氧化,抗菌,抗真菌和乙酰胆碱酯酶(AChE)抑制活性。通过FTIR,元素分析,ESR和HRMS阐明了化学结构。ESR光谱法和DFT计算证实,由于在溶液中形成了稳定的,以碳为中心的自由基阳离子,因此无法获得1 H和13 C NMR光谱。在体外使用四种测定法评估抗氧化剂效能:自由基清除活性(DPPH和ABTS),还原能力和总抗氧化剂容量测定法。BIP具有出色的抗氧化剂性能,并且在所有测定中,两种衍生物均被证明比参考抗氧化剂(抗坏血酸和Trolox)更有效。在ωB97XD/ 6-311 ++ g(d,p)水平上的DFT计算为BIP的自由基清除机理提供了有价值的见解。对于羟基取代的BIP,氢原子转移(HAT)是主要机制,而单电子转移与质子转移(SET-PT)结合则决定了其他衍生物的抗氧化活性。非共价相互作用分析和旋转势垒计算表明,分子内氢键(IHB)在抗氧化活性的机制中起着重要作用。从ESR分析获得的g因子和多重性。最有效的BIP对AChE表现出中等抑制活性(IC 50 = 20±4μM),而分子对接则表明在AChE的外围阴离子位点具有MMFF94结合焓为-43.4 kcal / mol的结合。已确定了BIP对几种致病细菌菌株的中等体外抗菌活性:铜绿假单胞菌,大肠杆菌,粪肠球菌,金黄色葡萄球菌和耐甲氧西林金黄色葡萄球菌(MRSA)的临床分离株。BIP对白念珠菌的抗真菌活性也被证实。结合分子对接的相似性集成方法表明,亮氨酰氨肽酶是三种最有效的BIP衍生物的可能抗微生物靶标。






























 京公网安备 11010802027423号
京公网安备 11010802027423号