当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catanionic Surfactant Self-Assembly in Protic Ionic Liquids.
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-07-06 , DOI: 10.1021/acs.jpclett.0c01608 Saffron J Bryant 1 , Rob Atkin 2 , Michael Gradzielski 3 , Gregory G Warr 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-07-06 , DOI: 10.1021/acs.jpclett.0c01608 Saffron J Bryant 1 , Rob Atkin 2 , Michael Gradzielski 3 , Gregory G Warr 1
Affiliation
|
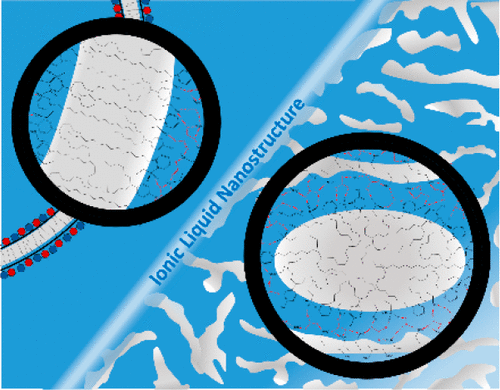
Mixing of cationic and anionic surfactants in water can result in pseudo-double-tailed catanionic surfactant ion pairs that form lamellar phases or vesicles that are unstable toward electrolyte addition. Here we show that despite the very high ionic strengths, catanionic surfactants counterintuitively form a wider variety of self-assembled aggregates in pure ionic liquids (ILs, pure salts in a liquid phase) than in water, including micelles, vesicles, and lyotropic phases. Self-assembled structures only form when the IL is sufficiently polar to drive self-assembly through electrostatic interactions and/or H-bond networks, but the catanionic effect is manifested only when the IL does not itself exhibit pronounced amphiphilic nanostructure. This enables the type of catanionic aggregate formed to be designed by changing the hydrogen bonds between the ions through variation of the structures of the cation and anion. These results reveal an entirely new way of controlling catanionic surfactant self-assembly under nonaqueous and high-salt conditions.
中文翻译:

质子离子液体中的阳离子表面活性剂的自组装。
阳离子和阴离子表面活性剂在水中的混合会导致伪双尾阳离子表面活性剂离子对,这些离子对形成层状相或囊泡,对电解质添加而言是不稳定的。在这里,我们显示,尽管离子强度很高,但阳离子表面活性剂在水和纯离子液体(ILs,液相纯盐)中比在水中(包括胶束,囊泡和溶致相)反直觉地形成了多种自组装聚集体。仅当IL具有足够的极性以通过静电相互作用和/或H键网络驱动自组装时,才会形成自组装结构,但是仅当IL自身不具有明显的两亲纳米结构时,才显示出阳离子作用。这使得可以通过改变阳离子和阴离子的结构来改变离子之间的氢键来设计形成的阳离子聚集体的类型。这些结果揭示了在非水和高盐条件下控制阳离子表面活性剂自组装的全新方法。
更新日期:2020-08-06
中文翻译:

质子离子液体中的阳离子表面活性剂的自组装。
阳离子和阴离子表面活性剂在水中的混合会导致伪双尾阳离子表面活性剂离子对,这些离子对形成层状相或囊泡,对电解质添加而言是不稳定的。在这里,我们显示,尽管离子强度很高,但阳离子表面活性剂在水和纯离子液体(ILs,液相纯盐)中比在水中(包括胶束,囊泡和溶致相)反直觉地形成了多种自组装聚集体。仅当IL具有足够的极性以通过静电相互作用和/或H键网络驱动自组装时,才会形成自组装结构,但是仅当IL自身不具有明显的两亲纳米结构时,才显示出阳离子作用。这使得可以通过改变阳离子和阴离子的结构来改变离子之间的氢键来设计形成的阳离子聚集体的类型。这些结果揭示了在非水和高盐条件下控制阳离子表面活性剂自组装的全新方法。





























 京公网安备 11010802027423号
京公网安备 11010802027423号