当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective Hydroboration and Hydrosilylation of N-Heteroarenes Catalyzed by a Zinc Alkyl Complex.
Organic Letters ( IF 4.9 ) Pub Date : 2020-07-06 , DOI: 10.1021/acs.orglett.0c02082 Xinxin Wang 1 , Yu Zhang 1 , Dan Yuan 1 , Yingming Yao 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-07-06 , DOI: 10.1021/acs.orglett.0c02082 Xinxin Wang 1 , Yu Zhang 1 , Dan Yuan 1 , Yingming Yao 1
Affiliation
|
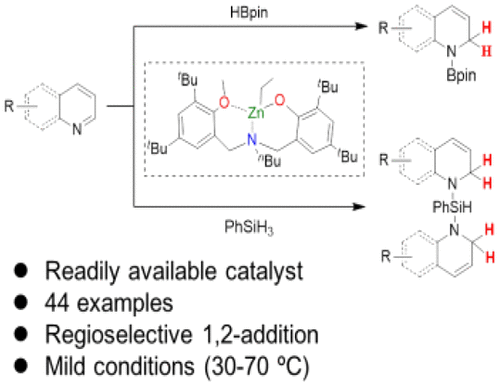
Readily available zinc alkyl complexes showed good activity and regioselectivity in catalyzing hydroboration and hydrosilylation of N-heteroarenes. Hydroboration of benzo-fused N-heterocycles gave exclusive 1,2-addition products in 80–97% yields. Reactions of pyridines afforded a mixture of 1,2-, 1,4-, and 1,6-products in yields of 55–95%, with 1,2-dihydropyridine as the main product. Bis-hydrosilylation was observed for quinoline derivatives, generating bis-1,2-hydrosilylation products in 76–96% yields. Kinetic studies and control experiments were conducted to gain some insights into the reaction mechanism.
中文翻译:

锌烷基络合物催化的N-杂芳烃的区域选择性加氢硼化和氢化硅烷化。
现成的烷基锌配合物在催化N-杂芳烃的硼氢化和氢化硅烷化反应中显示出良好的活性和区域选择性。苯并稠合的N-杂环的硼氢化生成独家的1,2-加成产物,产率为80-97%。吡啶反应得到1,2-,1,4-和1,6-产物的混合物,产率为55-95%,其中1,2-二氢吡啶为主要产物。观察到喹啉衍生物具有双氢化硅烷化作用,生成双1,2-氢化硅烷化产物的产率为76–96%。进行了动力学研究和对照实验,以期获得对反应机理的一些见解。
更新日期:2020-07-17
中文翻译:

锌烷基络合物催化的N-杂芳烃的区域选择性加氢硼化和氢化硅烷化。
现成的烷基锌配合物在催化N-杂芳烃的硼氢化和氢化硅烷化反应中显示出良好的活性和区域选择性。苯并稠合的N-杂环的硼氢化生成独家的1,2-加成产物,产率为80-97%。吡啶反应得到1,2-,1,4-和1,6-产物的混合物,产率为55-95%,其中1,2-二氢吡啶为主要产物。观察到喹啉衍生物具有双氢化硅烷化作用,生成双1,2-氢化硅烷化产物的产率为76–96%。进行了动力学研究和对照实验,以期获得对反应机理的一些见解。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号