当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Cyclopropanone Equivalents and Application to the Formation of Chiral β-Lactams.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-06 , DOI: 10.1002/anie.202006786 Christopher M Poteat 1 , Yujin Jang 1 , Myunggi Jung 1 , J Drake Johnson 1 , Rachel G Williams 1 , Vincent N G Lindsay 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-06 , DOI: 10.1002/anie.202006786 Christopher M Poteat 1 , Yujin Jang 1 , Myunggi Jung 1 , J Drake Johnson 1 , Rachel G Williams 1 , Vincent N G Lindsay 1
Affiliation
|
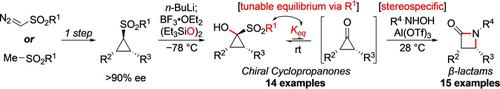
Cyclopropanone derivatives have long been considered unsustainable synthetic intermediates because of their extreme strain and kinetic instability. Reported here is the enantioselective synthesis of 1‐sulfonylcyclopropanols, as stable yet powerful equivalents of the corresponding cyclopropanone derivatives, by α‐hydroxylation of sulfonylcyclopropanes using a bis(silyl) peroxide as the electrophilic oxygen source. This work constitutes the first general approach to enantioenriched cyclopropanone derivatives. Both the electronic and steric nature of the sulfonyl moiety, which serves as a base‐labile protecting group and confers crystallinity to these cyclopropanone precursors, were found to have a crucial impact on the rate of equilibration to the corresponding cyclopropanone. The utility of these cyclopropanone surrogates is demonstrated in a mild and stereospecific formal [3+1] cycloaddition with simple hydroxylamines, leading to the efficient formation of chiral β‐lactam derivatives.
中文翻译:

对映体合成环丙烷酮当量及其在手性β-内酰胺形成中的应用。
长期以来,环丙烷酮衍生物一直被认为是不可持续的合成中间体,因为它们的极限应变和动力学不稳定。此处报道的是通过使用双(甲硅烷基)过氧化物作为亲电子氧源通过磺酰基环丙烷的α-羟基化反应,以对映选择性的方式合成1-磺酰基环丙醇,它是相应环丙烷酮衍生物的稳定而功能强大的当量。这项工作是对映体丰富的环丙烷酮衍生物的第一个通用方法。磺酰基部分的电子和空间性质都可以用作碱不稳定的保护基,并赋予这些环丙烷酮前体结晶性,这对平衡相应环丙烷酮的速率具有至关重要的影响。
更新日期:2020-07-06
中文翻译:

对映体合成环丙烷酮当量及其在手性β-内酰胺形成中的应用。
长期以来,环丙烷酮衍生物一直被认为是不可持续的合成中间体,因为它们的极限应变和动力学不稳定。此处报道的是通过使用双(甲硅烷基)过氧化物作为亲电子氧源通过磺酰基环丙烷的α-羟基化反应,以对映选择性的方式合成1-磺酰基环丙醇,它是相应环丙烷酮衍生物的稳定而功能强大的当量。这项工作是对映体丰富的环丙烷酮衍生物的第一个通用方法。磺酰基部分的电子和空间性质都可以用作碱不稳定的保护基,并赋予这些环丙烷酮前体结晶性,这对平衡相应环丙烷酮的速率具有至关重要的影响。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号