Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-07-06 , DOI: 10.1016/j.jhazmat.2020.123405 Qing Zheng 1 , Nannan Wu 2 , Ruijuan Qu 2 , Gadah Albasher 3 , Wanming Cao 2 , Beibei Li 2 , Nouf Alsultan 3 , Zunyao Wang 2
|
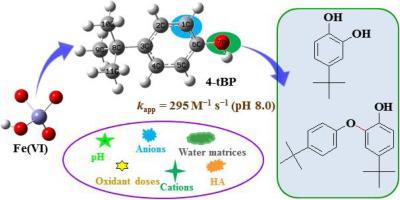
4-tert-butylphenol (4-tBP) is a phenolic endocrine disrupting chemical that has attracted great attention due to its wide occurrence, environmental persistence, and possible toxic effects. In this study, we systematically investigated the transformation of 4-tBP in ferrate (VI) oxidation process. The second-order reaction rate constant (kapp) of Fe(VI) with 4-tBP decreases with solution pH, and the kapp value was determined as 295 M−1·s−1 at pH 8.0. The removal efficiency of 4-tBP was slightly decreased by Mg2+ and HCO3–, while accelerated at varying degrees by the presence of Cu2+ and humic acid. Product analysis revealed that 4-tBP was mainly transformed into hydroxylation products, benzene-ring cleavage products, dimers and higher polymerization products via oxygen atom transfer, ring-opening of the benzene ring and radical coupling reaction. Furthermore, initial reactions of 4-tBP were rationalized by theoretical analysis of atom partial charges, frontier electron densities, and spin densities. Nearly complete removal of 4-tBP (20 μM) was achieved after 5 min of reaction in both ultrapure water and natural waters, demonstrating the feasibility of this Fe(VI) oxidation method in treating phenols-contaminated waters.
中文翻译:

高铁酸盐(VI)转化4-叔丁基苯酚的动力学和反应途径
4-叔丁基苯酚(4-tBP)是一种破坏酚类内分泌的化学物质,由于其广泛存在,环境持久性和可能的毒性作用而备受关注。在这项研究中,我们系统地研究了4-tBP在高铁酸盐(VI)氧化过程中的转化。Fe(VI)与4-tBP的二阶反应速率常数(k app)随溶液pH值降低,在pH 8.0时k app值确定为295 M -1 ·s -1。4-TBP的去除效率略有下降被Mg 2+和HCO 3 - ,而在由铜的存在不同程度的加速2+和腐殖酸。产物分析表明4-tBP主要通过氧原子转移,苯环开环和自由基偶联反应转变为羟基化产物,苯环裂解产物,二聚体和高级聚合产物。此外,通过对原子部分电荷,前沿电子密度和自旋密度的理论分析合理化了4-tBP的初始反应。在超纯水和天然水中反应5分钟后,几乎可以完全去除4-tBP(20μM),这证明了这种Fe(VI)氧化方法在处理受酚污染的水中的可行性。















































 京公网安备 11010802027423号
京公网安备 11010802027423号