当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Homologation of the Fischer Indolization: A Quinoline Synthesis via Homo-Diaza-Cope Rearrangement.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-04 , DOI: 10.1002/anie.202005798 Gabriela Guillermina Gerosa 1 , Sebastian Armin Schwengers 1 , Rajat Maji 1 , Chandra Kanta De 1 , Benjamin List 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-04 , DOI: 10.1002/anie.202005798 Gabriela Guillermina Gerosa 1 , Sebastian Armin Schwengers 1 , Rajat Maji 1 , Chandra Kanta De 1 , Benjamin List 1
Affiliation
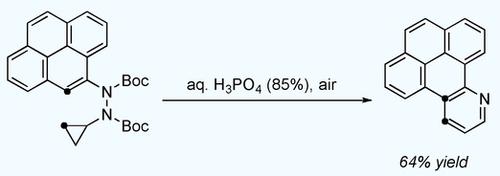
|
We disclose a new Brønsted acid promoted quinoline synthesis, proceeding via homo‐diaza‐Cope rearrangement of N‐aryl‐N′‐cyclopropyl hydrazines. Our strategy can be considered a homologation of Fischer's classical indole synthesis and delivers 6‐membered N‐heterocycles, including previously inaccessible pyridine derivatives. This approach can also be used as a pyridannulation methodology toward constructing polycyclic polyheteroaromatics. A computational analysis has been employed to probe plausible activation modes and to interrogate the role of the catalyst.
中文翻译:

Fischer 吲哚化的同系化:通过 Homo-Diaza-Cope 重排合成喹啉。
我们公开了一种新的布朗斯台德酸促进喹啉合成,通过N-芳基-N'-环丙基肼的同二氮杂-Cope 重排进行。我们的策略可以被认为是 Fischer 经典吲哚合成的同系物,并提供 6 元 N 杂环,包括以前无法获得的吡啶衍生物。该方法还可用作构建多环多杂芳烃的吡啶环化方法。已采用计算分析来探测合理的活化模式并询问催化剂的作用。
更新日期:2020-07-04
中文翻译:

Fischer 吲哚化的同系化:通过 Homo-Diaza-Cope 重排合成喹啉。
我们公开了一种新的布朗斯台德酸促进喹啉合成,通过N-芳基-N'-环丙基肼的同二氮杂-Cope 重排进行。我们的策略可以被认为是 Fischer 经典吲哚合成的同系物,并提供 6 元 N 杂环,包括以前无法获得的吡啶衍生物。该方法还可用作构建多环多杂芳烃的吡啶环化方法。已采用计算分析来探测合理的活化模式并询问催化剂的作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号