Materials Today Communications ( IF 3.7 ) Pub Date : 2020-07-04 , DOI: 10.1016/j.mtcomm.2020.101424 Amaranda García-Rodríguez , Sonia Merino , Julián Rodríguez-López
|
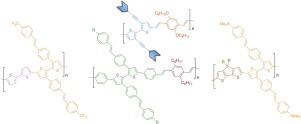
A variety of 2,2'-bithiophene derivatives with different π-conjugated arms at the 3,3'-positions have been synthesized and evaluated as suitable building blocks for the preparation of functional conjugated polymers. Polymerizations have been carried out through either Horner-Wadsworth-Emmons (HWE) or Stille cross coupling reactions with appropriate comonomers. Whereas the polymerization of 5,5'-diformyl- and 5,5'-dibromo-2,2'-bithiophenes did not extend beyond a small number of monomer units, 5,5'-bis(4-formylphenyl)-2,2'-bithiophenes formed polymers with high molecular weights, narrow polydispersity and good thermal stability. A preliminary study of the UV and photoluminescence properties of the prepared compounds is also presented.
中文翻译:

π共轭臂取代的2,2'-联噻吩的合成与聚合
合成了各种在3,3'-位置具有不同π-共轭臂的2,2'-联噻吩衍生物,并将其作为制备功能性共轭聚合物的合适结构单元进行了评估。聚合是通过霍纳-沃兹沃思-埃蒙斯(HWE)或斯蒂勒交叉偶联反应与合适的共聚单体进行的。尽管5,5'-二甲酰基-和5,5'-二溴-2,2'-联噻吩的聚合反应并未超出少数单体单元5,5'-双(4-甲酰基苯基)-2的聚合, 2'-联噻吩形成的聚合物具有高分子量,窄的多分散性和良好的热稳定性。还介绍了所制备化合物的紫外和光致发光性质的初步研究。






























 京公网安备 11010802027423号
京公网安备 11010802027423号