当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of (±)-Clivonine via Diels-Alder Reactions of 3,5-Dibromo-2-pyrone.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-01 , DOI: 10.1021/acs.joc.0c01283 Cheng-Dong Wang 1 , Qinyang Chen 1 , Seunghoon Shin 1 , Cheon-Gyu Cho 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-07-01 , DOI: 10.1021/acs.joc.0c01283 Cheng-Dong Wang 1 , Qinyang Chen 1 , Seunghoon Shin 1 , Cheon-Gyu Cho 1
Affiliation
|
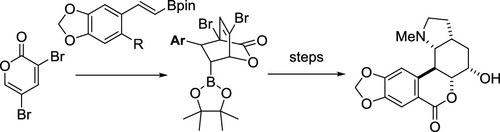
New synthetic routes to (±)-clivonine were devised starting with the Diels–Alder cycloadditions of 3,5-dibromo-2-pyrone with styrene pinacol boronate dienophiles. In the first-generation synthesis, the pivotal perhydroindoline system including the C5-hydroxyl group was constructed via a reaction sequence involving the Eschenmoser–Claisen rearrangement and regio/stereoselective epoxide opening reaction. In the second-generation synthesis, a radical-mediated cyclization approach was employed for the rapid assembly of the pyrrolidine ring. In this route, the C5-hydroxyl group provided by the dienophile in a stereochemically defined form was preserved throughout the synthesis.
中文翻译:

通过3,5-二溴-2-吡喃酮的Diels-Alder反应全合成(±)-君子碱。
从3,5-二溴-2-吡喃酮的狄尔斯-阿尔德与苯乙烯频哪醇硼酸酯二烯亲和体的狄尔斯-阿尔德环加成反应开始,设计了新的合成(±)-高佛宁碱的合成路线。在第一代合成中,通过涉及Eschenmoser-Claisen重排和区域/立体选择性环氧化物开放反应的反应序列构建了包括C5-羟基的关键过氢二氢吲哚啉系统。在第二代合成中,自由基介导的环化方法用于吡咯烷环的快速组装。在这种途径中,由亲双烯体以立体化学定义的形式提供的C5-羟基在整个合成过程中得以保留。
更新日期:2020-08-08
中文翻译:

通过3,5-二溴-2-吡喃酮的Diels-Alder反应全合成(±)-君子碱。
从3,5-二溴-2-吡喃酮的狄尔斯-阿尔德与苯乙烯频哪醇硼酸酯二烯亲和体的狄尔斯-阿尔德环加成反应开始,设计了新的合成(±)-高佛宁碱的合成路线。在第一代合成中,通过涉及Eschenmoser-Claisen重排和区域/立体选择性环氧化物开放反应的反应序列构建了包括C5-羟基的关键过氢二氢吲哚啉系统。在第二代合成中,自由基介导的环化方法用于吡咯烷环的快速组装。在这种途径中,由亲双烯体以立体化学定义的形式提供的C5-羟基在整个合成过程中得以保留。















































 京公网安备 11010802027423号
京公网安备 11010802027423号