Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding Adsorption Behaviors of Organic Friction Modifiers on Hydroxylated SiO2 (001) Surfaces: Effects of Molecular Polarity and Temperature.
Langmuir ( IF 3.7 ) Pub Date : 2020-07-02 , DOI: 10.1021/acs.langmuir.0c01386 Junqin Shi 1 , Qing Zhou 1 , Kun Sun 2 , Guoqiang Liu 1 , Feng Zhou 1, 3
Langmuir ( IF 3.7 ) Pub Date : 2020-07-02 , DOI: 10.1021/acs.langmuir.0c01386 Junqin Shi 1 , Qing Zhou 1 , Kun Sun 2 , Guoqiang Liu 1 , Feng Zhou 1, 3
Affiliation
|
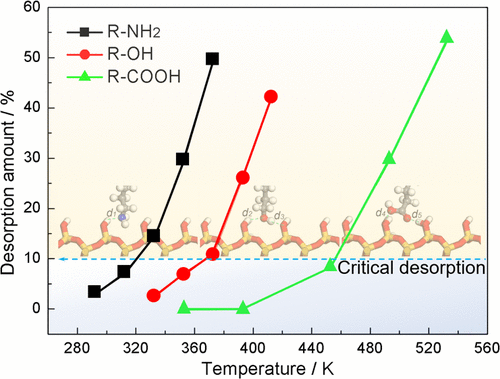
Molecular dynamics simulations are used to investigate the physisorption of organic friction modifiers (OFMs) lubricated by 1-decene trimer (PAO4) representing a base oil and confined between hydroxylated SiO2 (001) surfaces. The results indicate that OFM molecules form dense, tendentiously vertical monolayer films at low temperature but loose adsorption layers at high temperature, particularly for R-NH2 with weaker molecular polarity. The structural information is quantitatively clarified by mass density profiles, radial distribution function, and probability distributions of an end-to-end distance at a perpendicular-to-surface direction. The movement performance of lubricant, reflected by the thickness of the organic part and radius of gyration of PAO4 molecules, strongly depends on temperature. The adsorption amount of OFM molecules decreases dramatically with lowering OFM polarity and increasing temperature above the critical desorption temperatures of about 320, 373, and 453 K for amine (R-NH2), alcohol (R-OH), and acid (R-COOH), respectively. The interaction energies of the OFM-surface decrease continuously for the R-NH2 system with temperature and decrease rapidly as temperature exceeds a critical value for both R-OH and R-COOH systems. The single-molecule geometry optimization validates the significant role of the electrostatic and hydrogen-bond attractions in molecular adsorption. Therefore, the OFMs with stronger polarity (like R-COOH) present stronger adsorption and better temperature resistance. The findings in this work are of particular value and provide a guideline in designing and engineering novel OFM additives for extreme lubrication conditions.
中文翻译:

了解有机摩擦改性剂在羟基化SiO2(001)表面上的吸附行为:分子极性和温度的影响。
分子动力学模拟用于研究由1-癸烯三聚体(PAO4)润滑的有机摩擦改性剂(OFM)的物理吸附,该改性剂代表基础油并限制在羟基化SiO 2(001)表面之间。结果表明,OFM分子在低温下形成致密的,趋于垂直的单层膜,而在高温下则形成松散的吸附层,尤其是对于R-NH 2具有较弱的分子极性。通过质量密度分布,径向分布函数和垂直于表面方向上的端到端距离的概率分布来定量阐明结构信息。润滑剂的运动性能(取决于有机部分的厚度和PAO4分子的回转半径)在很大程度上取决于温度。当胺(R-NH 2),醇(R-OH)和酸(R-NH- )的临界解吸温度超过约320、373和453 K的临界解吸温度时,OFM分子的吸附量会随着OFM极性的降低和温度的升高而急剧下降。COOH)。对于R-NH 2,OFM表面的相互作用能不断降低R-OH和R-COOH系统的温度会随着温度超过临界值而迅速降低。单分子几何结构优化验证了静电和氢键吸引力在分子吸附中的重要作用。因此,极性更强的OFM(如R-COOH)表现出更强的吸附性和更好的耐热性。这项工作中的发现具有特殊价值,并为在极端润滑条件下设计和制造新型OFM添加剂提供了指导。
更新日期:2020-07-28
中文翻译:

了解有机摩擦改性剂在羟基化SiO2(001)表面上的吸附行为:分子极性和温度的影响。
分子动力学模拟用于研究由1-癸烯三聚体(PAO4)润滑的有机摩擦改性剂(OFM)的物理吸附,该改性剂代表基础油并限制在羟基化SiO 2(001)表面之间。结果表明,OFM分子在低温下形成致密的,趋于垂直的单层膜,而在高温下则形成松散的吸附层,尤其是对于R-NH 2具有较弱的分子极性。通过质量密度分布,径向分布函数和垂直于表面方向上的端到端距离的概率分布来定量阐明结构信息。润滑剂的运动性能(取决于有机部分的厚度和PAO4分子的回转半径)在很大程度上取决于温度。当胺(R-NH 2),醇(R-OH)和酸(R-NH- )的临界解吸温度超过约320、373和453 K的临界解吸温度时,OFM分子的吸附量会随着OFM极性的降低和温度的升高而急剧下降。COOH)。对于R-NH 2,OFM表面的相互作用能不断降低R-OH和R-COOH系统的温度会随着温度超过临界值而迅速降低。单分子几何结构优化验证了静电和氢键吸引力在分子吸附中的重要作用。因此,极性更强的OFM(如R-COOH)表现出更强的吸附性和更好的耐热性。这项工作中的发现具有特殊价值,并为在极端润滑条件下设计和制造新型OFM添加剂提供了指导。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号