当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of Gaseous Proteins via the Ion Evaporation Model (IEM) in Electrospray Mass Spectrometry.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-07-01 , DOI: 10.1021/acs.analchem.0c02290 Elnaz Aliyari 1 , Lars Konermann 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-07-01 , DOI: 10.1021/acs.analchem.0c02290 Elnaz Aliyari 1 , Lars Konermann 1
Affiliation
|
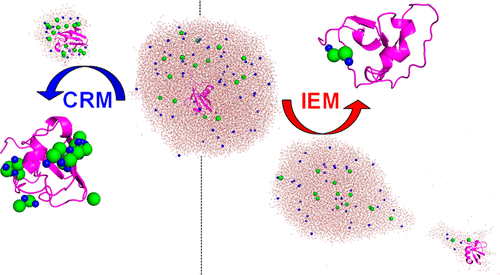
The mechanisms whereby protein ions are released into the gas phase from charged droplets during electrospray ionization (ESI) continue to be controversial. Several pathways have been proposed. For native ESI the charged residue model (CRM) is favored; it entails the liberation of proteins via solvent evaporation to dryness. Unfolded proteins likely follow the chain ejection model (CEM), which involves the gradual expulsion of stretched-out chains from the droplet. According to the ion evaporation model (IEM) ions undergo electrostatically driven desorption from the droplet surface. The IEM is well supported for small precharged species such as Na+. However, it is unclear whether proteins can show IEM behavior as well. We examined this question using molecular dynamics (MD) simulations, mass spectrometry (MS), and ion mobility spectrometry (IMS) in positive ion mode. Ubiquitin was chosen as the model protein because of its structural stability which allows the protein charge in solution to be controlled via pH adjustment without changing the protein conformation. MD simulations on small ESI droplets (3 nm radius) showed CRM behavior regardless of the protein charge in solution. Surprisingly, many MD runs on larger droplets (5.5 nm radius) culminated in IEM ejection of ubiquitin, as long as the protein carried a sufficiently large positive solution charge. MD simulations predicted that nonspecific salt adducts are less prevalent for IEM-generated protein ions than for CRM products. This prediction was confirmed experimentally. Also, collision cross sections of MD structures were in good agreement with IMS data. Overall, this work reveals that the CRM, CEM, and IEM all represent viable pathways for generating gaseous protein ions during ESI. The IEM is favored for proteins that are tightly folded and highly charged in solution and for droplets in a suitable size regime.
中文翻译:

通过电喷雾质谱中的离子蒸发模型(IEM)形成气态蛋白质。
在电喷雾电离(ESI)期间蛋白质离子从带电液滴释放到气相中的机制一直存在争议。已经提出了几种途径。对于原生ESI,倾向于使用带电残渣模型(CRM)。它需要通过溶剂蒸发至干燥来释放蛋白质。未折叠的蛋白质可能遵循链喷射模型(CEM),该模型涉及从液滴逐渐排出延伸的链。根据离子蒸发模型(IEM),离子会从液滴表面进行静电驱动的解吸。IEM可以很好地支持小型预充电物质,例如Na +。但是,尚不清楚蛋白质是否也可以显示IEM行为。我们在正离子模式下使用分子动力学(MD)模拟,质谱(MS)和离子迁移谱(IMS)检查了此问题。选择泛素作为模型蛋白是因为其结构稳定性,可以通过调节pH值来控制溶液中的蛋白电荷,而无需改变蛋白的构象。在小的ESI小滴(半径3 nm)上的MD模拟显示出CRM行为,而与溶液中的蛋白质电荷无关。出乎意料的是,只要蛋白质携带足够大的正溶液电荷,许多MD就会在较大的液滴(半径为5.5 nm)上运行,最终导致IEM泛素的喷射。MD模拟预测,IEM生成的蛋白质离子的非特异性盐加合物的发生率要低于CRM产品。这一预测在实验上得到了证实。而且,MD结构的碰撞截面与IMS数据非常吻合。总的来说,这项工作表明,CRM,CEM和IEM都代表了在ESI期间产生气态蛋白质离子的可行途径。IEM适用于紧密折叠并在溶液中带高电荷的蛋白质,以及适合大小范围的液滴。
更新日期:2020-08-04
中文翻译:

通过电喷雾质谱中的离子蒸发模型(IEM)形成气态蛋白质。
在电喷雾电离(ESI)期间蛋白质离子从带电液滴释放到气相中的机制一直存在争议。已经提出了几种途径。对于原生ESI,倾向于使用带电残渣模型(CRM)。它需要通过溶剂蒸发至干燥来释放蛋白质。未折叠的蛋白质可能遵循链喷射模型(CEM),该模型涉及从液滴逐渐排出延伸的链。根据离子蒸发模型(IEM),离子会从液滴表面进行静电驱动的解吸。IEM可以很好地支持小型预充电物质,例如Na +。但是,尚不清楚蛋白质是否也可以显示IEM行为。我们在正离子模式下使用分子动力学(MD)模拟,质谱(MS)和离子迁移谱(IMS)检查了此问题。选择泛素作为模型蛋白是因为其结构稳定性,可以通过调节pH值来控制溶液中的蛋白电荷,而无需改变蛋白的构象。在小的ESI小滴(半径3 nm)上的MD模拟显示出CRM行为,而与溶液中的蛋白质电荷无关。出乎意料的是,只要蛋白质携带足够大的正溶液电荷,许多MD就会在较大的液滴(半径为5.5 nm)上运行,最终导致IEM泛素的喷射。MD模拟预测,IEM生成的蛋白质离子的非特异性盐加合物的发生率要低于CRM产品。这一预测在实验上得到了证实。而且,MD结构的碰撞截面与IMS数据非常吻合。总的来说,这项工作表明,CRM,CEM和IEM都代表了在ESI期间产生气态蛋白质离子的可行途径。IEM适用于紧密折叠并在溶液中带高电荷的蛋白质,以及适合大小范围的液滴。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号