当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stabilizing the cationic/anionic redox chemistry of Li-rich layered cathodes by tuning the upper cut-off voltage for high energy-density lithium-ion batteries
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020-07-01 , DOI: 10.1039/d0ta05429a Peiyu Hou 1, 2, 3, 4, 5 , Feng Li 1, 2, 3, 4, 6 , Haiyan Zhang 4, 7, 8, 9 , Haitao Huang 4, 5, 10
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2020-07-01 , DOI: 10.1039/d0ta05429a Peiyu Hou 1, 2, 3, 4, 5 , Feng Li 1, 2, 3, 4, 6 , Haiyan Zhang 4, 7, 8, 9 , Haitao Huang 4, 5, 10
Affiliation
|
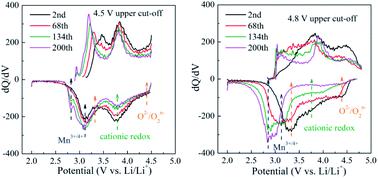
Cumulative cationic/anionic redox reactions boost the reversible capacity of Li-rich layered oxides (LLOs) to 300 mA h g−1. However, the voltage/capacity fade is a major obstacle to the application of LLOs in lithium-ion batteries (LIBs). Here a paradigm shift toward tuning the charging voltages, namely a low upper cut-off voltage of 4.5 V (vs. Li/Li+) after an initial activation at 4.6 V, is proposed and the effects of cut-off voltages (4.8 V and 4.5 V) on the stability of cationic/anionic redox chemistries along with structural evolution are also revealed. It is demonstrated that the reversibility of cationic/anionic redox processes can be significantly improved at the low cut-off voltage compared with that at 4.8 V. The structural transitions from the layered to the rock-salt phase along with the valence decrease of Mn ions are consequently mitigated. As a result, the LLOs exhibit a very low capacity/voltage fade of only 4.8%/7.8% after 200 cycles at 0.5C and superior rate capability (195 mA h g−1 at 5C). Additionally, full cells composed of the LLO cathode and a mesocarbon microbead anode show a high energy density exceeding 300 W h kg−1 and improved cycling/voltage stability. This strategy provides a simple and efficient method to stabilize the cationic/anionic redox chemistries of LLOs and thus promotes their practical applications in high energy-density LIBs.
中文翻译:

通过调整高能量密度锂离子电池的上限电压来稳定富锂层状阴极的阳离子/阴离子氧化还原化学
累积的阳离子/阴离子氧化还原反应将富锂层氧化物(LLO)的可逆容量提高到300 mA hg -1。但是,电压/容量衰减是将LLO应用于锂离子电池(LIB)的主要障碍。在这里,模式向着调整充电电压方向转变,即4.5 V的低上限电压(vs. Li / Li +)在4.6 V初始激活后,提出了该方法,并揭示了截止电压(4.8 V和4.5 V)对阳离子/阴离子氧化还原化学稳定性以及结构演变的影响。结果表明,与4.8 V相比,在低截止电压下,阳离子/阴离子氧化还原过程的可逆性得到了显着改善。从层状相到岩盐相的结构转变以及Mn离子的化合价降低因此得到缓解。结果,LLO在0.5C的200个循环后表现出非常低的电容/电压衰减,仅为4.8%/ 7.8%,并且具有出色的速率能力(在5C时为195 mA hg -1)。此外,由LLO阴极和中碳微珠阳极组成的全电池显示出超过300 W h kg -1的高能量密度并改善了循环/电压稳定性。该策略提供了一种简单有效的方法来稳定LLO的阳离子/阴离子氧化还原化学,从而促进了它们在高能量密度LIB中的实际应用。
更新日期:2020-07-21
中文翻译:

通过调整高能量密度锂离子电池的上限电压来稳定富锂层状阴极的阳离子/阴离子氧化还原化学
累积的阳离子/阴离子氧化还原反应将富锂层氧化物(LLO)的可逆容量提高到300 mA hg -1。但是,电压/容量衰减是将LLO应用于锂离子电池(LIB)的主要障碍。在这里,模式向着调整充电电压方向转变,即4.5 V的低上限电压(vs. Li / Li +)在4.6 V初始激活后,提出了该方法,并揭示了截止电压(4.8 V和4.5 V)对阳离子/阴离子氧化还原化学稳定性以及结构演变的影响。结果表明,与4.8 V相比,在低截止电压下,阳离子/阴离子氧化还原过程的可逆性得到了显着改善。从层状相到岩盐相的结构转变以及Mn离子的化合价降低因此得到缓解。结果,LLO在0.5C的200个循环后表现出非常低的电容/电压衰减,仅为4.8%/ 7.8%,并且具有出色的速率能力(在5C时为195 mA hg -1)。此外,由LLO阴极和中碳微珠阳极组成的全电池显示出超过300 W h kg -1的高能量密度并改善了循环/电压稳定性。该策略提供了一种简单有效的方法来稳定LLO的阳离子/阴离子氧化还原化学,从而促进了它们在高能量密度LIB中的实际应用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号