当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tetracoordinate borates as catalysts for reductive formylation of amines with carbon dioxide
Green Chemistry ( IF 9.3 ) Pub Date : 2020-07-01 , DOI: 10.1039/d0gc01741h
Xiaolin Jiang 1, 2, 3, 4, 5 , Zijun Huang 1, 2, 3, 4, 5 , Mohamed Makha 1, 2, 3, 4, 5 , Chen-Xia Du 6, 7, 8, 9 , Dongmei Zhao 9, 10, 11, 12 , Fang Wang 1, 2, 3, 4, 5 , Yuehui Li 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2020-07-01 , DOI: 10.1039/d0gc01741h
Xiaolin Jiang 1, 2, 3, 4, 5 , Zijun Huang 1, 2, 3, 4, 5 , Mohamed Makha 1, 2, 3, 4, 5 , Chen-Xia Du 6, 7, 8, 9 , Dongmei Zhao 9, 10, 11, 12 , Fang Wang 1, 2, 3, 4, 5 , Yuehui Li 1, 2, 3, 4, 5
Affiliation
|
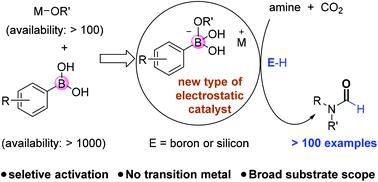
We report sodium trihydroxyaryl borates as the first robust tetracoordinate organoboron catalysts for reductive functionalization of CO2. These catalysts, easily synthesized from condensing boronic acids with metal hydroxides, activate main group element–hydrogen (E–H) bonds efficiently. In contrast to BX3 type boranes, boronic acids and metal-BAr4 salts, under transition metal-free conditions, sodium trihydroxyaryl borates exhibit high reactivity of reductive N-formylation toward a variety of amines (106 examples), including those with functional groups such as ester, olefin, hydroxyl, cyano, nitro, halogen, MeS–, ether groups, etc. The over-performance to catalyze formylation of challenging pyridyl amines affords a promising alternative method to the use of traditional formylation reagents. Mechanistic investigation supports electrostatic interactions as the key for Si/B–H activation, enabling alkali metal borates as versatile catalysts for hydroborylation, hydrosilylation, and reductive formylation/methylation of CO2.
中文翻译:

四配位硼酸酯作为胺与二氧化碳还原甲酰化的催化剂
我们报告三羟基芳基硼酸钠作为第一个坚固的四配位有机硼催化剂,用于CO 2的还原功能化。这些催化剂很容易从硼酸与金属氢氧化物的缩合反应中合成,可以有效地活化主族元素氢键。与BX 3型硼烷,硼酸和金属-BAr 4盐相比,在无过渡金属的条件下,三羟基芳基硼酸钠对各种胺(包括带有官能团的胺)表现出较高的还原性N-甲酰化反应性(106例)例如酯,烯烃,羟基,氰基,硝基,卤素,MeS–,醚基等。催化具有挑战性的吡啶胺的甲酰化反应的性能过高,为使用传统的甲酰化试剂提供了一种有前途的替代方法。机理研究支持将静电相互作用作为Si / B–H活化的关键,从而使碱金属硼酸盐成为用于CO 2加氢硼化,加氢硅烷化和还原甲酰化/甲基化的通用催化剂。
更新日期:2020-08-18
中文翻译:

四配位硼酸酯作为胺与二氧化碳还原甲酰化的催化剂
我们报告三羟基芳基硼酸钠作为第一个坚固的四配位有机硼催化剂,用于CO 2的还原功能化。这些催化剂很容易从硼酸与金属氢氧化物的缩合反应中合成,可以有效地活化主族元素氢键。与BX 3型硼烷,硼酸和金属-BAr 4盐相比,在无过渡金属的条件下,三羟基芳基硼酸钠对各种胺(包括带有官能团的胺)表现出较高的还原性N-甲酰化反应性(106例)例如酯,烯烃,羟基,氰基,硝基,卤素,MeS–,醚基等。催化具有挑战性的吡啶胺的甲酰化反应的性能过高,为使用传统的甲酰化试剂提供了一种有前途的替代方法。机理研究支持将静电相互作用作为Si / B–H活化的关键,从而使碱金属硼酸盐成为用于CO 2加氢硼化,加氢硅烷化和还原甲酰化/甲基化的通用催化剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号