当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Making and Breaking Leupeptin Protease Inhibitors in Pathogenic Gammaproteobacteria.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-01 , DOI: 10.1002/anie.202005506 Jhe-Hao Li 1, 2 , Joonseok Oh 1, 2 , Sabine Kienesberger 3 , Nam Yoon Kim 1, 2 , David J Clarke 4 , Ellen L Zechner 3, 5 , Jason M Crawford 1, 2, 6
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-07-01 , DOI: 10.1002/anie.202005506 Jhe-Hao Li 1, 2 , Joonseok Oh 1, 2 , Sabine Kienesberger 3 , Nam Yoon Kim 1, 2 , David J Clarke 4 , Ellen L Zechner 3, 5 , Jason M Crawford 1, 2, 6
Affiliation
|
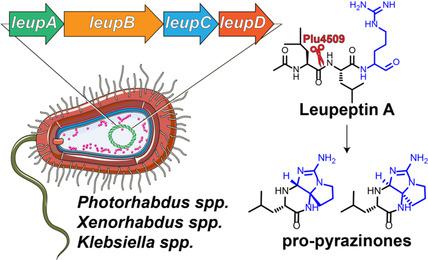
Leupeptin is a bacterial small molecule that is used worldwide as a protease inhibitor. However, its biosynthesis and genetic distribution remain unknown. We identified a family of leupeptins in gammaproteobacterial pathogens, including Photorhabdus, Xenorhabdus, and Klebsiella species, amongst others. Through genetic, metabolomic, and heterologous expression analyses, we established their construction by discretely expressed ligases and accessory enzymes. In Photorhabdus species, a hypothetical protein required for colonizing nematode hosts was established as a new class of proteases. This enzyme cleaved the tripeptide aldehyde protease inhibitors, leading to the formation of “pro‐pyrazinones” featuring a hetero‐tricyclic architecture. In Klebsiella oxytoca, the pathway was enriched in clinical isolates associated with respiratory tract infections. Thus, the bacterial production and proteolytic degradation of leupeptins can be associated with animal colonization phenotypes.
中文翻译:

在致病性丙型杆菌中制造和破坏亮肽素蛋白酶抑制剂。
亮肽素是一种细菌性小分子,在世界范围内被用作蛋白酶抑制剂。然而,其生物合成和遗传分布仍然未知。我们确定了γ变形杆菌病原体中的亮肽素家族,包括Photorhabdus,Xenorhabdus和Klebsiella物种。通过遗传,代谢组和异源表达分析,我们通过离散表达的连接酶和辅助酶建立了它们的构建。在Photorhabdus物种,建立线虫宿主所需的一种假定蛋白质被确立为一类新的蛋白酶。该酶裂解了三肽醛蛋白酶抑制剂,导致形成具有杂三环结构的“原吡嗪酮”。在产酸克雷伯菌中,该途径富含与呼吸道感染相关的临床分离株。因此,亮肽素的细菌产生和蛋白水解降解可以与动物定殖表型有关。
更新日期:2020-07-01
中文翻译:

在致病性丙型杆菌中制造和破坏亮肽素蛋白酶抑制剂。
亮肽素是一种细菌性小分子,在世界范围内被用作蛋白酶抑制剂。然而,其生物合成和遗传分布仍然未知。我们确定了γ变形杆菌病原体中的亮肽素家族,包括Photorhabdus,Xenorhabdus和Klebsiella物种。通过遗传,代谢组和异源表达分析,我们通过离散表达的连接酶和辅助酶建立了它们的构建。在Photorhabdus物种,建立线虫宿主所需的一种假定蛋白质被确立为一类新的蛋白酶。该酶裂解了三肽醛蛋白酶抑制剂,导致形成具有杂三环结构的“原吡嗪酮”。在产酸克雷伯菌中,该途径富含与呼吸道感染相关的临床分离株。因此,亮肽素的细菌产生和蛋白水解降解可以与动物定殖表型有关。































 京公网安备 11010802027423号
京公网安备 11010802027423号