Pharmacological Research ( IF 9.1 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.phrs.2020.105059
Qi Gao 1 , Ai Wei 1 , Fang Chen 1 , Xingren Chen 1 , Wenwen Ding 1 , Zhiquan Ding 1 , Zhiwei Wu 1 , Ronghui Du 1 , Wangsen Cao 1
|
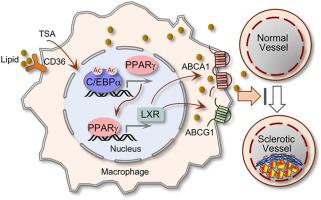
Atherosclerosis (AS) is a risky cardiovascular disease with limited treatment options. Various pan or type-selective histone deacetylase (HDAC) inhibitors are reportedly atheroprotective against atherosclerosis (AS); however, the key effectors and the main cellular processes that mediate the protective effects remain poorly defined. Here, we report that PPARγ (Peroxisome proliferator-activated receptor gamma), a transcription factor actively involved in lipid metabolism with strong tissue protective and anti-inflammation properties, is a critical mediator of the anti-AS effects by HDAC inhibition. We showed that a well-known pan-HDAC inhibitor TSA (Trichostatin A) reduced foam cell formation of macrophages that is accompanied by a marked elevation of PPARγ and its downstream cholesterol efflux transporter ABCA1 (ATP-binding membrane cassette transport protein A1) and ABCG1. In an AS model of ApoE−/− mice fed on high-fat diet, TSA treatment alleviated AS lesions, similarly increased PPARγ and the downstream cholesterol transporters and mitigated the induction of inflammatory cytokine TNFα and IL-1β. Exploring the potential cause of PPARγ elevation revealed that TSA induced the acetylation of C/EBPα (CCAAT enhancer binding protein alpha), the upstream regulator of PPARγ, through which it increased PPARγ transactivation. More importantly, we generated a strain of PPARγ/ApoE double knockout mice and demonstrated that lack of PPARγ abrogated the protective effects of TSA on foam cell formation of peritoneal macrophages and the AS pathogenesis. Taken together, these results unravel that C/EBPα and PPARγ are the HDAC-sensitive components of an epigenetic signaling pathway mediating foam cell formation and AS development, and suggest that targeting C/EBPα/PPARγ axis by HDAC inhibitors possesses therapeutic potentials in retarding the progression of AS and the related cardiovascular diseases.
中文翻译:

通过 HDAC 抑制增强 PPARγ 可减少 ApoE 缺陷小鼠的泡沫细胞形成和动脉粥样硬化。
动脉粥样硬化 (AS) 是一种危险的心血管疾病,治疗选择有限。据报道,各种泛型或类型选择性组蛋白脱乙酰酶 (HDAC) 抑制剂对动脉粥样硬化 (AS) 具有动脉粥样硬化保护作用;然而,介导保护作用的关键效应物和主要细胞过程仍然不清楚。在这里,我们报告了 PPARγ(过氧化物酶体增殖物激活受体 γ),一种积极参与脂质代谢的转录因子,具有强大的组织保护和抗炎特性,是 HDAC 抑制抗 AS 效应的关键介质。我们发现,众所周知的 pan-HDAC 抑制剂 TSA(曲古抑菌素 A)减少了巨噬细胞的泡沫细胞形成,伴随着 PPARγ 及其下游胆固醇流出转运蛋白 ABCA1(ATP 结合膜盒转运蛋白 A1)和 ABCG1 的显着升高. 在 ApoE 的 AS 模型中-/-在高脂肪饮食喂养的小鼠中,TSA 治疗减轻了 AS 病变,同样增加了 PPARγ 和下游胆固醇转运蛋白,并减轻了炎性细胞因子 TNFα 和 IL-1β 的诱导。探索 PPARγ 升高的潜在原因表明,TSA 诱导了 PPARγ 上游调节因子 C/EBPα(CCAAT 增强子结合蛋白 α)的乙酰化,从而增加了 PPARγ 反式激活。更重要的是,我们产生了一株 PPARγ/ApoE 双基因敲除小鼠,并证明缺乏 PPARγ 消除了 TSA 对腹膜巨噬细胞泡沫细胞形成和 AS 发病机制的保护作用。总之,这些结果表明 C/EBPα 和 PPARγ 是介导泡沫细胞形成和 AS 发展的表观遗传信号通路的 HDAC 敏感成分,































 京公网安备 11010802027423号
京公网安备 11010802027423号