当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic Restructuring Induced Oxygen Activation on AgCu Near-Surface Alloys.
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-06-30 , DOI: 10.1021/acs.jpclett.0c00887 Laura A. Cramer , Yilang Liu , Prashant Deshlahra , E. Charles H. Sykes
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-06-30 , DOI: 10.1021/acs.jpclett.0c00887 Laura A. Cramer , Yilang Liu , Prashant Deshlahra , E. Charles H. Sykes
|
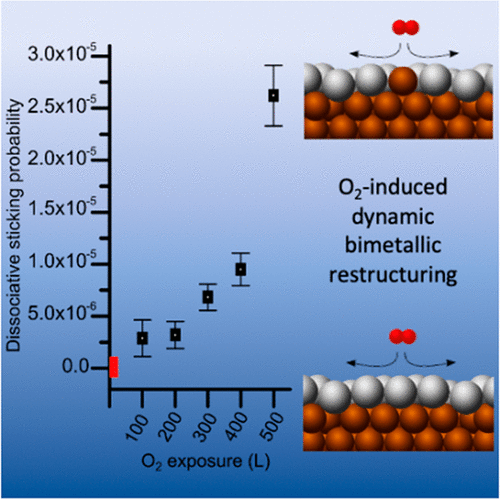
Recent studies have shown that the addition of Cu to Ag catalysts improves their epoxidation performance by increasing the overall selectivity of the bimetallic catalyst. We have prepared AgCu near-surface alloys and used scanning tunneling microscopy to gain an atomistic picture of O2 dissociation on the bimetallic system. These data reveal a higher dissociative sticking probability for O2 on AgCu than on Ag(111), and density functional theory (DFT) confirms that the O2 dissociation barrier is 0.17 eV lower on the alloy. Surprisingly, we find that, after a slow initial uptake of O2, the sticking probability increases exponentially. Further DFT calculations indicate that surface oxygen reverses the segregation energy for AgCu, stabilizing Cu atoms in the Ag layer. These single Cu atoms in the Ag surface are found to significantly lower the O2 dissociation barrier. Together, these results explain nonlinear effects in the activation of O2 on this catalytically relevant surface alloy.
中文翻译:

动态重组在AgCu近表面合金上诱导氧活化。
最近的研究表明,向Ag催化剂中添加Cu可通过提高双金属催化剂的整体选择性来改善其环氧化性能。我们已经制备了AgCu近表面合金,并使用扫描隧道显微镜获得了双金属体系上O 2离解的原子图。这些数据表明,O 2在AgCu上的离解粘附几率高于在Ag(111)上,并且密度泛函理论(DFT)证实,O 2在合金上的解离势垒低0.17 eV。令人惊讶的是,我们发现,在缓慢吸收初始O 2之后,粘着几率成倍增加。进一步的DFT计算表明,表面氧逆转了AgCu的偏析能,从而稳定了Ag层中的Cu原子。发现Ag表面中的这些单个Cu原子显着降低了O 2的解离势垒。总之,这些结果解释了在该催化相关表面合金上O 2活化中的非线性效应。
更新日期:2020-08-06
中文翻译:

动态重组在AgCu近表面合金上诱导氧活化。
最近的研究表明,向Ag催化剂中添加Cu可通过提高双金属催化剂的整体选择性来改善其环氧化性能。我们已经制备了AgCu近表面合金,并使用扫描隧道显微镜获得了双金属体系上O 2离解的原子图。这些数据表明,O 2在AgCu上的离解粘附几率高于在Ag(111)上,并且密度泛函理论(DFT)证实,O 2在合金上的解离势垒低0.17 eV。令人惊讶的是,我们发现,在缓慢吸收初始O 2之后,粘着几率成倍增加。进一步的DFT计算表明,表面氧逆转了AgCu的偏析能,从而稳定了Ag层中的Cu原子。发现Ag表面中的这些单个Cu原子显着降低了O 2的解离势垒。总之,这些结果解释了在该催化相关表面合金上O 2活化中的非线性效应。































 京公网安备 11010802027423号
京公网安备 11010802027423号