当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Contributions of Experimental Data Obtained in Concentrated Mixtures to Kinetic Studies: Application to Monomethylhydrazine Pyrolysis.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-06-30 , DOI: 10.1021/acs.jpca.0c03144 Pascal Diévart 1 , Laurent Catoire 1, 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-06-30 , DOI: 10.1021/acs.jpca.0c03144 Pascal Diévart 1 , Laurent Catoire 1, 2
Affiliation
|
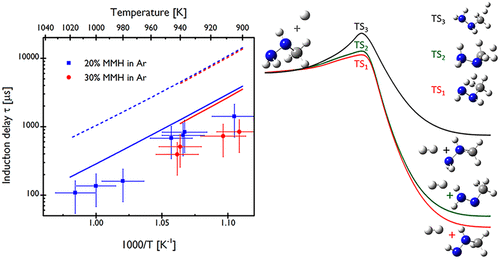
Experimental, numerical, and theoretical studies are performed to understand the explosive thermal decomposition of monomethylhydrazine/argon mixtures. Ignition delays of concentrated MMH/Ar mixtures (20–30%) have been measured behind a reflected shock wave around 1000 K and 1 atm. Although several detailed chemical kinetic models have predictive abilities for diluted and highly diluted mixtures, none of them showed predictive for concentrated mixtures. A new kinetic model is proposed, in which numerous rate constants and thermochemical data are reassessed based on theoretical calculations, with the purpose to determine whether, or to what extent, trends derived from diluted or highly diluted MMH/Ar mixtures can explain observations in concentrated MMH mixtures. The present kinetic model is found to predict speciation experimental profiles in diluted MMH/Ar mixtures and is a significant improvement in predicting the induction delays of concentrated MMH/Ar mixtures.
中文翻译:

在浓缩混合物中获得的实验数据对动力学研究的贡献:在单甲基肼热解中的应用。
进行了实验,数值和理论研究,以了解单甲基肼/氩气混合物的爆炸性热分解。在大约1000 K和1 atm的反射冲击波后,测量了浓MMH / Ar混合物(20–30%)的点火延迟。尽管一些详细的化学动力学模型对稀释和高度稀释的混合物具有预测能力,但没有一个对浓缩混合物具有预测能力。提出了一种新的动力学模型,其中基于理论计算重新评估了许多速率常数和热化学数据,目的是确定稀释或高度稀释的MMH / Ar混合物产生的趋势是否可以解释或在多大程度上可以解释浓缩中的观察结果MMH混合物。
更新日期:2020-07-30
中文翻译:

在浓缩混合物中获得的实验数据对动力学研究的贡献:在单甲基肼热解中的应用。
进行了实验,数值和理论研究,以了解单甲基肼/氩气混合物的爆炸性热分解。在大约1000 K和1 atm的反射冲击波后,测量了浓MMH / Ar混合物(20–30%)的点火延迟。尽管一些详细的化学动力学模型对稀释和高度稀释的混合物具有预测能力,但没有一个对浓缩混合物具有预测能力。提出了一种新的动力学模型,其中基于理论计算重新评估了许多速率常数和热化学数据,目的是确定稀释或高度稀释的MMH / Ar混合物产生的趋势是否可以解释或在多大程度上可以解释浓缩中的观察结果MMH混合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号