当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Catalytic Role of a Conserved Tyrosine in Nitric Oxide-Reducing Non-heme Diiron Enzymes
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-30 , DOI: 10.1021/acscatal.0c01884 Saborni Biswas 1 , Donald M. Kurtz 2 , Samuel R. Montoya 2 , Michael P. Hendrich 1 , Emile L. Bominaar 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-30 , DOI: 10.1021/acscatal.0c01884 Saborni Biswas 1 , Donald M. Kurtz 2 , Samuel R. Montoya 2 , Michael P. Hendrich 1 , Emile L. Bominaar 1
Affiliation
|
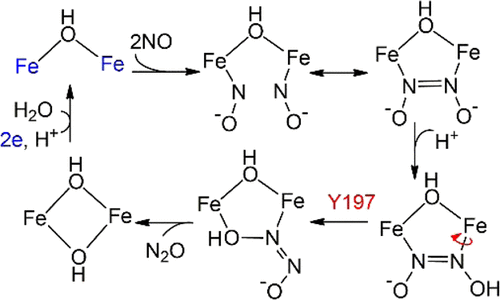
Recent studies have identified several key intermediates of the nitric oxide reductase (NOR) cycle of flavodiiron proteins (FDPs). These intermediates include, sequentially, a μ-hydroxo diferrous species, a mono-NO intermediate, a di-NO intermediate (FDPdiNO), and a bis-μ-hydroxo diferric species. This paper focuses on the reaction path connecting the last two intermediates on which the nitrous oxide product is released. On the basis of density functional theory calculations, a reaction sequence is proposed in which the enzyme passes through an intermediate with a bridging hyponitrite ligand, which accepts a proton, initiating a rate-determining ligand rotation from an N/N- to an N/O-coordinated conformation. This rotation is facilitated by a second-sphere tyrosine residue, which provides a transient hydrogen bond to one of the nitrogen atoms of the substrate near the transition state. The role of the tyrosine residue in the NOR activity has been tested by steady-state kinetics and rapid freeze-quench (RFQ) studies of the Y197F variant of the FDP from T. maritima in which the hydrogen bonding interaction is absent. The Y197F variant displayed little or no steady-state NOR activity in support of the importance of Y197. The RFQ samples, monitored by Mössbauer spectroscopy, showed that Y197F follows the same reaction path as a wild-type FDP up to and including the formation of FDPdiNO but diverges subsequently with the variant forming an inactive mono-NO species. The RFQ results demonstrate that Y197 enables the postFDPdiNO section of the reaction cycle in the wild-type FDP to proceed to N2O. The proposed refinement of the reaction mechanism provides an explanation for the lack of NOR activity of the variant Y197F of T. maritima and of other di-NO binding diiron enzymes and model compounds with active site structures like those of FDPs. The reported reductions of NO to N2O catalyzed by synthetic diiron complexes proceed only with the support of radiation, additional electrons, or an electron-rich ligand environment. However, higher potential diiron sites like those of FDPs require hydrogen bonding to second-sphere residues to turn over NO to N2O.
中文翻译:

保守的酪氨酸在还原一氧化氮的非血红素二铁酶中的催化作用
最近的研究已经确定了黄酮二铁蛋白(FDPs)的一氧化氮还原酶(NOR)循环的几个关键中间体。这些中间体依次包括μ-羟基二亚铁物种,单-NO中间体,双-NO中间体(FDP diNO)和bis-μ-hydroxo二铁物种。本文关注于连接最后两个中间体的反应路径,在该中间体上释放出一氧化二氮产物。在密度泛函理论计算的基础上,提出了一种反应顺序,其中酶穿过带有桥接次亚硝酸盐配体的中间体,该中间体接受质子,从而启动速率决定配体从N / N-到N /的旋转。 O坐标构象。第二球形酪氨酸残基促进了这种旋转,该残基在过渡态附近向底物的一个氮原子提供了一个瞬态氢键。酪氨酸残基在NOR活性中的作用已通过稳态动力学和快速冷冻猝灭(RFQ)研究来验证,该研究来自海螯虾的FDP的Y197F变体。其中不存在氢键相互作用。Y197F变体显示很少或没有稳态NOR活性,以支持Y197的重要性。由穆斯堡尔光谱法监测的RFQ样品显示,Y197F遵循与野生型FDP相同的反应路径,直至FDP diNO形成并包括FDP diNO形成,但随后发生变异,形成了非活性单NO物种。询价结果表明,Y197使postFDP DINO在野生型FDP反应循环的部分继续到N 2 O的所提出的反应机理的改进方案提供了缺少的NOR活性的变体Y197F的说明Ť马里蒂玛以及具有活性位点结构的其他二氧化氮结合二铁酶和模型化合物,例如FDP。报道的合成二铁配合物催化的NO还原为N 2 O仅在辐射,附加电子或富电子配体环境的支持下进行。然而,较高的潜在二铁等网站那些FDP项目的需要氢键第二球残基为NO翻身为N 2 O.
更新日期:2020-08-08
中文翻译:

保守的酪氨酸在还原一氧化氮的非血红素二铁酶中的催化作用
最近的研究已经确定了黄酮二铁蛋白(FDPs)的一氧化氮还原酶(NOR)循环的几个关键中间体。这些中间体依次包括μ-羟基二亚铁物种,单-NO中间体,双-NO中间体(FDP diNO)和bis-μ-hydroxo二铁物种。本文关注于连接最后两个中间体的反应路径,在该中间体上释放出一氧化二氮产物。在密度泛函理论计算的基础上,提出了一种反应顺序,其中酶穿过带有桥接次亚硝酸盐配体的中间体,该中间体接受质子,从而启动速率决定配体从N / N-到N /的旋转。 O坐标构象。第二球形酪氨酸残基促进了这种旋转,该残基在过渡态附近向底物的一个氮原子提供了一个瞬态氢键。酪氨酸残基在NOR活性中的作用已通过稳态动力学和快速冷冻猝灭(RFQ)研究来验证,该研究来自海螯虾的FDP的Y197F变体。其中不存在氢键相互作用。Y197F变体显示很少或没有稳态NOR活性,以支持Y197的重要性。由穆斯堡尔光谱法监测的RFQ样品显示,Y197F遵循与野生型FDP相同的反应路径,直至FDP diNO形成并包括FDP diNO形成,但随后发生变异,形成了非活性单NO物种。询价结果表明,Y197使postFDP DINO在野生型FDP反应循环的部分继续到N 2 O的所提出的反应机理的改进方案提供了缺少的NOR活性的变体Y197F的说明Ť马里蒂玛以及具有活性位点结构的其他二氧化氮结合二铁酶和模型化合物,例如FDP。报道的合成二铁配合物催化的NO还原为N 2 O仅在辐射,附加电子或富电子配体环境的支持下进行。然而,较高的潜在二铁等网站那些FDP项目的需要氢键第二球残基为NO翻身为N 2 O.































 京公网安备 11010802027423号
京公网安备 11010802027423号