Letters in Drug Design & Discovery ( IF 1.2 ) Pub Date : 2020-05-31 , DOI: 10.2174/1570180816666190710142848 Shi-Ben Wang 1 , Hui Liu 2 , Guang-Yong Li 1 , Kang Lei 1 , Xiao-Jing Li 1 , Zhe-Shan Quan 3 , Xue-Kun Wang 1
|
Background: Although Antiepileptic Drugs (AEDs) acting on various targets have been applied in the clinic, the efficacy and tolerance of AEDs in the treatment of epilepsy have not significantly improved. Therefore, there is an urgent need to develop some novel chemical moieties with a better safety profile and greater efficacy. We designed and synthesized twenty-seven 4- phenylpiperidin-2-one derivatives. This study aimed to investigate the potential use of a series of 4- phenylpiperidin-2-one derivatives as anticonvulsant drugs.
Methods: Two experimental methods, Maximal Electroshock (MES) and subcutaneous pentylenetetrazole (scPTZ), were used to evaluate the anticonvulsant activity of the target compounds. Moreover, neurotoxicity (NT) was tested using the rotarod test.
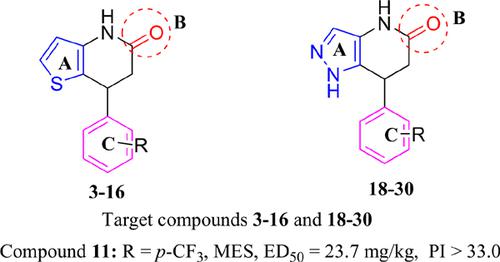
Results: Compound 7-[4-(trifluoromethyl)phenyl]-6,7-dihydrothieno[3,2-b]pyridin-5-(4H)-one (11; MES, ED50 = 23.7 mg/kg, PI > 33.7; PTZ, ED50 = 78.1 mg/kg, PI > 10.0) showed the best anticonvulsant activity. The results of in vivo γ-aminobutyric Acid (GABA) estimation showed that compound 11 may have an effect on the GABA system. Compound 11 showed significant interactions with residues at the benzodiazepine (BZD)-binding site on GABAA receptors. Most target compounds have favorable blood brain barrier (BBB) permeability and oral bioavailability in predictions using silico molecular properties.
Conclusion: According to the in vivo and in silico studies, compound 11 stand out as potential anticonvulsant agents for further studies.
中文翻译:

4-苯基哌啶-2-酮衍生物的合成及其抗惊厥活性
背景:尽管在临床上已应用了作用于各种靶标的抗癫痫药物(AED),但AED在治疗癫痫病中的功效和耐受性并未得到明显改善。因此,迫切需要开发一些具有更好安全性和更高功效的新型化学部分。我们设计并合成了27个4-苯基哌啶-2-酮衍生物。这项研究旨在调查一系列4-苯基哌啶-2-酮衍生物作为抗惊厥药物的潜在用途。
方法:采用两种实验方法,最大电击(MES)和皮下戊四氮(scPTZ)评估目标化合物的抗惊厥活性。此外,使用旋转脚踏试验测试了神经毒性(NT)。
结果:化合物7- [4-(三氟甲基)苯基] -6,7-二氢噻吩并[3,2-b]吡啶-5-(4H)-一(11; MES,ED50 = 23.7 mg / kg,PI> 33.7 ; PTZ,ED50 = 78.1 mg / kg,PI> 10.0)显示出最佳的抗惊厥活性。体内γ-氨基丁酸(GABA)估计结果表明,化合物11可能对GABA系统有影响。化合物11与GABAA受体上的苯二氮杂(BZD)结合位点的残基表现出显着的相互作用。使用硅胶分子特性进行预测时,大多数目标化合物具有良好的血脑屏障(BBB)渗透性和口服生物利用度。
结论:根据体内和计算机模拟研究,化合物11作为潜在的抗惊厥药值得进一步研究。






























 京公网安备 11010802027423号
京公网安备 11010802027423号