当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
From Salt in Solution to Solely Ions: Solvation of Methyl Viologen in Deep Eutectic Solvents and Ionic Liquids.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-26 , DOI: 10.1021/acs.jpcb.0c03296 Jeffrey M Klein 1 , Henry Squire 1 , William Dean 1 , Burcu E Gurkan 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-06-26 , DOI: 10.1021/acs.jpcb.0c03296 Jeffrey M Klein 1 , Henry Squire 1 , William Dean 1 , Burcu E Gurkan 1
Affiliation
|
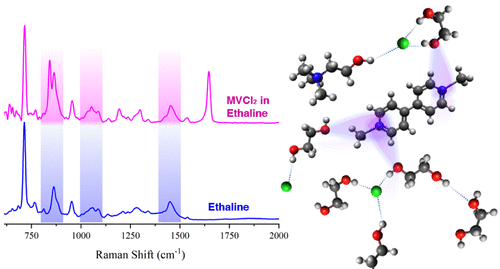
Solvation and transport properties of methly viologen dichloride (MVCl2) in 1:2, 1:4, and 1:6 molar mixtures of choline chloride (ChCl) and ethylene glycol (EG), including the deep eutectic solvent (DES) ethaline (1:2 mixture), were studied through the application of the hole theory to measured physical properties, cyclic voltammetry, and Raman spectroscopy. The ChCl:EG mixtures were compared to the ionic liquid (IL) 1-methyl-1-propylpyrrolidinium bis(trifluoromethylsulfonyl) imide ([PYR13][TFSI]) and choline bis(trifluoromethylsulfonyl)imide (ChTFSI) EG mixtures with the same molar ratios in order to understand the impact of the anion and hydrogen bond donor on solvation. Exchanging the chloride anion with TFSI is found to increase the fluidity of the solvent and promote stronger solute–solvent interactions. Raman spectroscopy suggests MVCl2 is strongly solvated by EG in ChTFSI:EG solutions and interstitially accommodated in holes in ChCl:EG mixtures and [PYR13][TFSI]. Complex solvents such as ILs and DESs are regarded as “designer solvents”, and it is demonstrated here that the physical properties and solvation characteristics of these fluids strongly depend on the choice of the anion.
中文翻译:

从溶液中的盐到纯离子:深共晶溶剂和离子液体中的甲基紫精的溶剂化。
氯化胆碱(ChCl)和乙二醇(EG)的1:2、1:4和1:6摩尔混合物中的二氯甲基紫罗兰(MVCl 2)的溶剂化和输运特性,包括深共熔溶剂(DES)乙胺(通过将空穴理论应用于测量的物理性质,循环伏安法和拉曼光谱研究了1:2混合物)。将ChCl:EG混合物与离子液体(IL)1-甲基-1-丙基吡咯烷鎓双(三氟甲基磺酰基)酰亚胺([PYR 13] [TFSI])和胆碱双(三氟甲基磺酰基)酰亚胺(ChTFSI)EG摩尔比相同的混合物,以了解阴离子和氢键供体对溶剂化的影响。发现用TFSI交换氯离子可增加溶剂的流动性并促进更强的溶质-溶剂相互作用。拉曼光谱表明,MVCl 2被EG在ChTFSI:EG溶液中强烈溶解,并被间隙地容纳在ChCl:EG混合物和[PYR 13 ] [TFSI]的孔中。复杂的溶剂(例如ILs和DESs)被视为“设计溶剂”,并且在此证明这些流体的物理性质和溶剂化特性强烈取决于阴离子的选择。
更新日期:2020-07-23
中文翻译:

从溶液中的盐到纯离子:深共晶溶剂和离子液体中的甲基紫精的溶剂化。
氯化胆碱(ChCl)和乙二醇(EG)的1:2、1:4和1:6摩尔混合物中的二氯甲基紫罗兰(MVCl 2)的溶剂化和输运特性,包括深共熔溶剂(DES)乙胺(通过将空穴理论应用于测量的物理性质,循环伏安法和拉曼光谱研究了1:2混合物)。将ChCl:EG混合物与离子液体(IL)1-甲基-1-丙基吡咯烷鎓双(三氟甲基磺酰基)酰亚胺([PYR 13] [TFSI])和胆碱双(三氟甲基磺酰基)酰亚胺(ChTFSI)EG摩尔比相同的混合物,以了解阴离子和氢键供体对溶剂化的影响。发现用TFSI交换氯离子可增加溶剂的流动性并促进更强的溶质-溶剂相互作用。拉曼光谱表明,MVCl 2被EG在ChTFSI:EG溶液中强烈溶解,并被间隙地容纳在ChCl:EG混合物和[PYR 13 ] [TFSI]的孔中。复杂的溶剂(例如ILs和DESs)被视为“设计溶剂”,并且在此证明这些流体的物理性质和溶剂化特性强烈取决于阴离子的选择。































 京公网安备 11010802027423号
京公网安备 11010802027423号