当前位置:
X-MOL 学术
›
ChemNanoMat
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural, defect, transport and dopant properties of AgNbO3
ChemNanoMat ( IF 2.6 ) Pub Date : 2020-07-15 , DOI: 10.1002/cnma.202000327 Navaratnarajah Kuganathan 1, 2 , Alexander Chroneos 1, 2
ChemNanoMat ( IF 2.6 ) Pub Date : 2020-07-15 , DOI: 10.1002/cnma.202000327 Navaratnarajah Kuganathan 1, 2 , Alexander Chroneos 1, 2
Affiliation
|
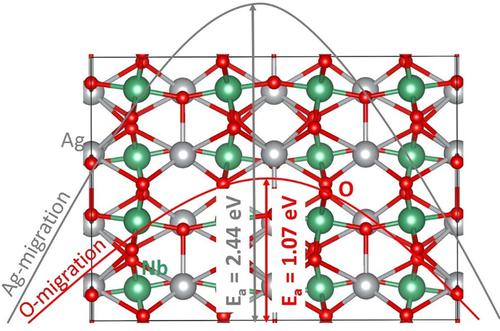
Silver niobate (AgNbO3) is a candidate lead‐free piezoelectric materials with potential applications in electronic technology and catalysis. Atomistic simulation techniques are used to examine the defects, diffusion of Ag+ and O2− ions, solution of dopants and electronic structures of pristine and doped configurations in AgNbO3. The Ag Frenkel is the most favourable intrinsic defect leading to the formation of Ag vacancies that can vehicle self‐diffusion of Ag+ ions in this material. The calculated activation energy for the diffusion of O2− ions (1.07 eV) is significantly lower than that calculated for the diffusion of Ag+ ions (2.44 eV). The prominent isovalent dopants on the Ag and the Nb sites are found to be Na+ and Ta5+ respectively. Doping of Ge on the Nb site can facilitate the formation of oxygen vacancies required for the oxygen diffusion. Additional Ag vacancies required for the self‐diffusion of silver can be introduced by doping of Ca on the Ag site. Electronic structures of non‐defective and defective AgNbO3 are discussed using density functional theory calculations.
中文翻译:

AgNbO3的结构,缺陷,传输和掺杂特性
铌酸银(AgNbO 3)是一种候选的无铅压电材料,在电子技术和催化领域具有潜在的应用前景。原子模拟技术用于检查AgNbO 3中的缺陷,Ag +和O 2-离子的扩散,掺杂剂的溶液以及原始的电子结构和掺杂构型。Ag Frenkel是最有利的内在缺陷,可导致Ag空位的形成,该空位可导致Ag +离子在该材料中自扩散。计算出的O 2−离子扩散的活化能(1.07 eV)明显低于计算Ag +扩散的活化能离子(2.44 eV)。发现在Ag和Nb位点上突出的等价掺杂剂分别是Na +和Ta 5+。在Nb部位掺杂Ge可以促进形成氧扩散所需的氧空位。银自身扩散所需的其他Ag空位可通过在Ag部位掺杂Ca来引入。使用密度泛函理论计算讨论了无缺陷和有缺陷的AgNbO 3的电子结构。
更新日期:2020-07-15
中文翻译:

AgNbO3的结构,缺陷,传输和掺杂特性
铌酸银(AgNbO 3)是一种候选的无铅压电材料,在电子技术和催化领域具有潜在的应用前景。原子模拟技术用于检查AgNbO 3中的缺陷,Ag +和O 2-离子的扩散,掺杂剂的溶液以及原始的电子结构和掺杂构型。Ag Frenkel是最有利的内在缺陷,可导致Ag空位的形成,该空位可导致Ag +离子在该材料中自扩散。计算出的O 2−离子扩散的活化能(1.07 eV)明显低于计算Ag +扩散的活化能离子(2.44 eV)。发现在Ag和Nb位点上突出的等价掺杂剂分别是Na +和Ta 5+。在Nb部位掺杂Ge可以促进形成氧扩散所需的氧空位。银自身扩散所需的其他Ag空位可通过在Ag部位掺杂Ca来引入。使用密度泛函理论计算讨论了无缺陷和有缺陷的AgNbO 3的电子结构。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号