Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2020-06-26 , DOI: 10.1016/j.bioorg.2020.104041 Yi-Ming Li 1 , Rong-Hua Luo 2 , Liu-Meng Yang 2 , Si-Ming Huang 1 , Sui-Yuan Li 1 , Yu-Gui Zheng 2 , Dong-Xuan Ni 1 , Yi-Man Cui 1 , Xing-Jie Zhang 1 , Xiao-Li Li 1 , Rui-Han Zhang 1 , E Tang 1 , Hong-Bin Zhang 1 , Yong-Tang Zheng 2 , Yan-Ping He 1 , Wei-Lie Xiao 1
|
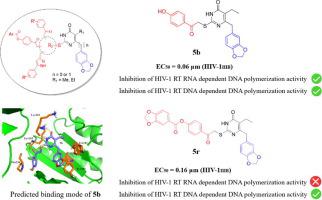
In order to discover and develop the new HIV-1 NNRTIs, a series of 5-alkyl-6-(benzo[d][1,3]dioxol-5-ylalkyl)-2-mercaptopyrimidin-4(3H)-ones was synthesized and screened for their in vitro cytotoxicity against HIV-1. Most of the compounds we synthetized showed high activity against wild-type HIV-1 strain (IIIB) while IC50 values are in the range of 0.06–12.95 μM. Among them, the most active HIV-1 inhibitor was compound 6-(benzo[d][1,3]dioxol-5-ylmethyl)-5-ethyl-2-((2-(4-hydroxyphenyl)-2-oxoethyl)thio)pyrimidin-4(3H)-one (5b), which exhibited similar HIV-1 inhibitory potency (IC50 = 0.06 μM, CC50 = 96.23 μM) compared with nevirapine (IC50 = 0.04 μM, CC50 >200 μM) and most of compounds exhibited submicromolar IC50 values indicating they were specific RT inhibitors. The compounds 5b, 6-(benzo[d] [1,3]dioxol-5-yl)-5-ethyl-2-((2-(4-hydroxyphenyl)-2-oxoethyl)thio)pyrimidin-4(3H)-one (5c) and 4-(2-((4-(benzo[d][1,3]dioxol-5-ylmethyl)-5-ethyl-6-oxo-1,6-dihydropyrimidin-2-yl)thio)acetyl)phenylbenzo[d][1,3]dioxole-5-carboxylate (5r) were selected for further study. It was found that all of them had little toxicity to peripheral blood mononuclear cell (PBMC), and had a good inhibitory effect on the replication of HIV-1 protease inhibitor resistant strains, fusion inhibitor resistant strains and nucleosides reverse transcriptase inhibitor resistant strains, as well as on clinical isolates. Besides, compound 5b and 5c showed inhibition of HIV-1 RT RNA-dependent DNA polymerization activity and DNA-dependent DNA polymerization activity, while compound 5r only showed inhibition of HIV DNA-dependent DNA polymerization activity, which was different from classical reverse transcriptase inhibitors. Our study which offered the preliminary structure-activity relationships and modeling studies of these new compounds has provided the valuable avenues for future molecular optimization.
中文翻译:

设计,合成和抗HIV评估作为有效HIV-1 NNRTIs的5-烷基-6-(苯并[d] [1,3]二氧杂-5-烷基)-2-巯基嘧啶-4(3H)-。
为了发现和开发新的HIV-1 NNRTIs,制备了一系列5-烷基-6-(苯并[d] [1,3]二氧杂-5-基烷基)-2-巯基嘧啶-4(3H)-。合成并筛选它们对HIV-1的体外细胞毒性。我们合成的大多数化合物显示出对野生型HIV-1菌株(IIIB)的高活性,而IC 50值在0.06-12.95μM的范围内。其中,最具活性的HIV-1抑制剂是化合物6-(苯并[d] [1,3]二氧杂-5-基甲基)-5-乙基-2-(((2-(4-羟基苯基)-2-氧乙基) )硫基嘧啶4(3H)-一(5b) 与奈韦拉平(IC 50 = 0.04μM,CC )表现出相似的HIV-1抑制力(IC 50 = 0.06μM,CC 50 = 96.23μM)50 > 200μM),大多数化合物的亚微摩尔IC 50值表明它们是特定的RT抑制剂。化合物5b,6-(苯并[d] [1,3]二氧杂-5-基)-5-乙基-2-((2-(4-羟基苯基)-2-氧乙基)硫基)嘧啶-4(3H)- (5c)和4-(2-((4-(苯并[d] [1,3]二氧戊-5-基甲基)-5-乙基-6-氧代-1,6-二氢嘧啶-2-基)硫基)乙酰基)苯基苯并[d] [1,3]二恶唑-5-羧酸酯(5r)被选作进一步研究。发现它们对外周血单个核细胞(PBMC)几乎没有毒性,并且对HIV-1蛋白酶抑制剂抗性菌株,融合抑制剂抗性菌株和核苷逆转录酶抑制剂抗性菌株的复制具有良好的抑制作用,如以及临床分离株。此外,化合物5b和5c抑制HIV-1 RT RNA依赖性DNA聚合活性和DNA依赖性DNA聚合活性,而化合物5r仅显示出对HIV DNA依赖性DNA聚合活性的抑制,这与经典的逆转录酶抑制剂不同。我们的研究提供了这些新化合物的初步结构-活性关系和模型研究,为将来的分子优化提供了有价值的途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号