Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2020-06-26 , DOI: 10.1016/j.bbapap.2020.140480
Tomoki Nakayoshi 1 , Koichi Kato 2 , Eiji Kurimoto 3 , Akifumi Oda 4
|
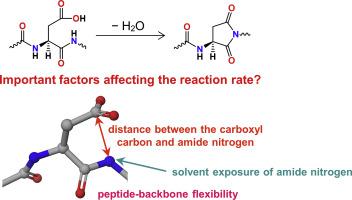
The isomerization rate of aspartic acid (Asp) residue is known to be affected by the three-dimensional structures of peptides and proteins. Although the isomerized Asp residues were experimentally observed, structural features which affect the isomerization cannot be elucidated sufficiently because of protein denaturation and aggregation. In this study, molecular dynamics (MD) simulations were conducted on three αA-crystallin peptides (T6, T10, and T18), each containing a single Asp residue with different isomerization rate (T18 > T6 > T10) to clarify the structural factors of Asp isomerization tendency. For MD trajectories, distances between side-chain carboxyl carbon of Asp and main-chain amide nitrogen of (n + 1) residue (Cγ–N distances), root mean square fluctuations (RMSFs), and polar surface areas for main-chain amide nitrogen of (n + 1) residues (PSAN) were calculated, because these structural features are considered to relate to the formations of cyclic imide intermediates. RMSFs and PSAN are indexes of peptide backbone flexibilities and solvent exposure of the amide nitrogen, respectively. The average Cγ–N distances of T10 was longer than those of the other two peptides. In addition, the peptide containing Asp residue with a higher isomerization rate showed higher flexibility of the peptide backbone around the Asp residue. PSAN for amide nitrogen in T18 were much larger than those of other two peptides. The computational results suggest that Asp-residue isomerization rates are affected by these factors.
中文翻译:

αA-晶状蛋白肽的构象对天冬氨酸残基异构化速率的影响。
已知天冬氨酸(Asp)残基的异构化速率受肽和蛋白质的三维结构影响。尽管已通过实验观察到异构化的Asp残基,但由于蛋白质的变性和聚集作用,无法充分阐明影响异构化的结构特征。在这项研究中,对三种αA-晶状体蛋白肽(T6,T10和T18)进行了分子动力学(MD)模拟,每种肽都包含具有不同异构化速率(T18> T6> T10)的单个Asp残基,以阐明天冬氨酸异构化趋势。对于MD轨迹,Asp的侧链羧基碳与(n +1)个残基(Cγ)的主链酰胺氮之间的距离–N距离),均方根波动(RMSFs)和(n + 1)个残基的主链酰胺氮的极性表面积(PSA N),因为这些结构特征被认为与环的形成有关酰亚胺中间体。RMSF和PSA N分别是肽主链柔韧性和酰胺氮溶剂暴露的指标。T10的平均Cγ– N距离比其他两种肽更长。另外,具有更高异构化速率的含有Asp残基的肽显示出Asp残基周围的肽主链具有较高的柔性。PSA NT18中的酰胺氮比其他两个肽大得多。计算结果表明,Asp残基异构化速率受这些因素影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号