当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mg3(BH4)4(NH2)2 as Inorganic Solid Electrolyte with High Mg2+ Ionic Conductivity
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-06-24 00:00:00 , DOI: 10.1021/acsaem.0c00980 Ronan Le Ruyet 1, 2 , Benoît Fleutot 1, 2 , Romain Berthelot 2, 3 , Yasmine Benabed 4, 5 , Geoffroy Hautier 4 , Yaroslav Filinchuk 4 , Raphaël Janot 1, 2
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2020-06-24 00:00:00 , DOI: 10.1021/acsaem.0c00980 Ronan Le Ruyet 1, 2 , Benoît Fleutot 1, 2 , Romain Berthelot 2, 3 , Yasmine Benabed 4, 5 , Geoffroy Hautier 4 , Yaroslav Filinchuk 4 , Raphaël Janot 1, 2
Affiliation
|
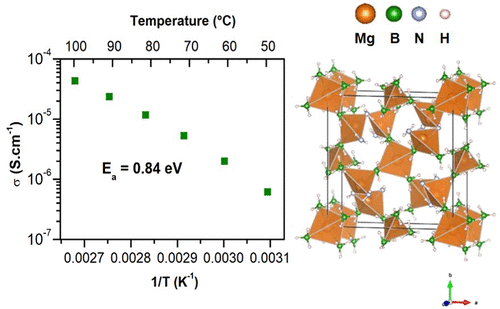
Mg3(BH4)4(NH2)2 compound was synthesized through the investigation of the Mg(BH4)2-Mg(NH2)2 phase diagram; its crystal structure was solved in a tetragonal unit cell with the space group I4̅. Interestingly, Mg3(BH4)4(NH2)2 has a high thermal stability with a decomposition temperature above 190 °C and exhibits a high Mg2+ ionic conductivity of 4.1 × 10–5 S·cm–1 at 100 °C with a low activation energy (0.84 eV). The reversible Mg deposition/stripping was demonstrated at 100 °C when using Mg3(BH4)4(NH2)2 as solid electrolyte. Thus, Mg3(BH4)4(NH2)2 is a compound that could help to develop rechargeable Mg-ion solid-state batteries.
中文翻译:

Mg 3(BH 4)4(NH 2)2作为具有高Mg 2+离子电导率的无机固体电解质
通过研究Mg(BH 4)2 -Mg(NH 2)2相图,合成了Mg 3(BH 4)4(NH 2)2化合物;其晶体结构与所述空间群的四方单元电池解决了我4。有趣的是,Mg 3(BH 4)4(NH 2)2具有较高的热稳定性,分解温度高于190°C,并且具有4.1×10 –5 S·cm –1的高Mg 2+离子电导率。在100°C下具有较低的活化能(0.84 eV)。当使用Mg 3(BH 4)4(NH 2)2作为固体电解质时,在100°C时可逆的Mg沉积/剥离被证明。因此,Mg 3(BH 4)4(NH 2)2是有助于开发可充电Mg离子固态电池的化合物。
更新日期:2020-06-24
中文翻译:

Mg 3(BH 4)4(NH 2)2作为具有高Mg 2+离子电导率的无机固体电解质
通过研究Mg(BH 4)2 -Mg(NH 2)2相图,合成了Mg 3(BH 4)4(NH 2)2化合物;其晶体结构与所述空间群的四方单元电池解决了我4。有趣的是,Mg 3(BH 4)4(NH 2)2具有较高的热稳定性,分解温度高于190°C,并且具有4.1×10 –5 S·cm –1的高Mg 2+离子电导率。在100°C下具有较低的活化能(0.84 eV)。当使用Mg 3(BH 4)4(NH 2)2作为固体电解质时,在100°C时可逆的Mg沉积/剥离被证明。因此,Mg 3(BH 4)4(NH 2)2是有助于开发可充电Mg离子固态电池的化合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号