Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Genetically Encoded Split-Luciferase Biosensors to Measure Endosome Disruption Rapidly in Live Cells.
ACS Sensors ( IF 8.2 ) Pub Date : 2020-06-23 , DOI: 10.1021/acssensors.0c00103 Kameron V Kilchrist 1 , John William Tierney 1 , Craig L Duvall 1
ACS Sensors ( IF 8.2 ) Pub Date : 2020-06-23 , DOI: 10.1021/acssensors.0c00103 Kameron V Kilchrist 1 , John William Tierney 1 , Craig L Duvall 1
Affiliation
|
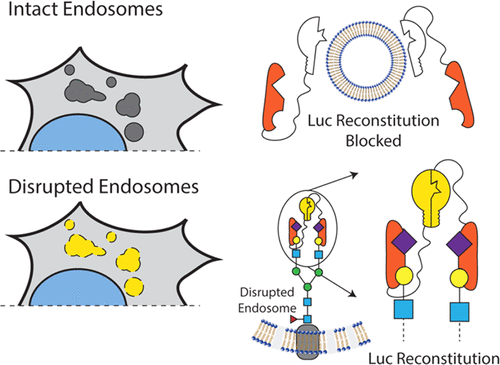
Endosomal escape is a critical step in the intracellular delivery of biomacromolecular drugs, but a quantitative, high-throughput study of endosomal-vesicle disruption remains elusive. We designed two genetically encoded split-luciferase turn-on reporter assays that can be measured rapidly in well plates on live cells using a luminometer. Both systems use nonluminescent N-terminal and C-terminal luciferase fragments that can reconstitute a functional luminescent enzyme when they are colocalized by their fusion partners. The first system uses luciferase-fragment fusion to Galectin 8 (Gal8) and CALCOCO2. Gal8 and CALCOCO2 interact following endosomal-vesicle disruption to facilitate luciferase complementation into the active enzyme, enabling a luminescence readout (G8C2 system). The second system expresses the N-terminal carbohydrate recognition domain (N-CRD) of Gal8 fused to each luciferase fragment (G8G8 system). Following endosome disruption, G8-NCRD binds to exposed glycans inside endosomes, concentrating both fragments in close proximity and reconstituting active luciferase. The G8G8 system emerged as the lead reporter candidate and was further characterized by comparing it to previously reported Gal8-YFP tracking using microscopy. We also characterized the G8G8 system response to several commercial and research drug-delivery reagents: DOTAP lipid, JetPEI, Lipofectamine 2000, and a library of polymers with known endosomal-escape activity, revealing dose-dependent increases in luminescence due to endosomal disruption. These new reporters provide a first-in-class luminescent assay to rapidly detect endosome disruption in a high-throughput format while excluding toxic formulations. Endosome-disruption screening with these turn-on assays has the potential to accelerate and to improve the rigor of programs focused on the discovery and development of intracellular biologic drug-delivery formulations.
中文翻译:

基因编码的拆分荧光素酶生物传感器,可快速测量活细胞中的内体破坏。
内体逃逸是生物大分子药物向细胞内递送的关键步骤,但是对内体囊泡破坏的定量,高通量研究仍然遥遥无期。我们设计了两种遗传编码的分裂荧光素酶开启报告基因测定法,可以使用发光计在活细胞的孔板上快速测量。两种系统都使用不发光的N端和C端荧光素酶片段,当它们被融合伴侣共定位时,它们可以重新构建功能性的发光酶。第一个系统使用萤光素酶片段融合至Galectin 8(Gal8)和CALCOCO2。内体囊泡破坏后,Gal8和CALCOCO2相互作用以促进荧光素酶与活性酶互补,从而实现发光读数(G8C2系统)。第二个系统表达与每个荧光素酶片段融合的Gal8的N端碳水化合物识别结构域(N-CRD)(G8G8系统)。内体破坏后,G8-NCRD与内体内部暴露的聚糖结合,将两个片段紧密结合并重组活性荧光素酶。G8G8系统成为主要的报告基因候选物,并且通过与使用显微镜检查的先前报道的Gal8-YFP跟踪进行比较来进一步表征。我们还对G8G8系统对几种商业和研究药物递送试剂的反应进行了表征:DOTAP脂质,JetPEI,Lipofectamine 2000以及具有已知内体逃逸活性的聚合物库,揭示了由于内体破坏而导致的剂量依赖性发光增加。这些新的报道分子提供了一流的发光测定法,可以快速检测高通量形式的内体破坏,同时排除有毒制剂。使用这些开启检测方法进行的内体破坏筛选具有加速和提高针对发现和开发细胞内生物药物传递制剂的程序的严格性的潜力。
更新日期:2020-07-24
中文翻译:

基因编码的拆分荧光素酶生物传感器,可快速测量活细胞中的内体破坏。
内体逃逸是生物大分子药物向细胞内递送的关键步骤,但是对内体囊泡破坏的定量,高通量研究仍然遥遥无期。我们设计了两种遗传编码的分裂荧光素酶开启报告基因测定法,可以使用发光计在活细胞的孔板上快速测量。两种系统都使用不发光的N端和C端荧光素酶片段,当它们被融合伴侣共定位时,它们可以重新构建功能性的发光酶。第一个系统使用萤光素酶片段融合至Galectin 8(Gal8)和CALCOCO2。内体囊泡破坏后,Gal8和CALCOCO2相互作用以促进荧光素酶与活性酶互补,从而实现发光读数(G8C2系统)。第二个系统表达与每个荧光素酶片段融合的Gal8的N端碳水化合物识别结构域(N-CRD)(G8G8系统)。内体破坏后,G8-NCRD与内体内部暴露的聚糖结合,将两个片段紧密结合并重组活性荧光素酶。G8G8系统成为主要的报告基因候选物,并且通过与使用显微镜检查的先前报道的Gal8-YFP跟踪进行比较来进一步表征。我们还对G8G8系统对几种商业和研究药物递送试剂的反应进行了表征:DOTAP脂质,JetPEI,Lipofectamine 2000以及具有已知内体逃逸活性的聚合物库,揭示了由于内体破坏而导致的剂量依赖性发光增加。这些新的报道分子提供了一流的发光测定法,可以快速检测高通量形式的内体破坏,同时排除有毒制剂。使用这些开启检测方法进行的内体破坏筛选具有加速和提高针对发现和开发细胞内生物药物传递制剂的程序的严格性的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号