当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dirhodium(II)‐Catalyzed Cyclopropanation of Alkyne‐Containing α‐Diazoacetates for the Synthesis of Cycloalkynes
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-22 , DOI: 10.1002/adsc.202000360 Qian Zeng 1 , Ciwang He 1 , Su Zhou 2 , Kuiyong Dong 1 , Lihua Qiu 1 , Xinfang Xu 1, 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-22 , DOI: 10.1002/adsc.202000360 Qian Zeng 1 , Ciwang He 1 , Su Zhou 2 , Kuiyong Dong 1 , Lihua Qiu 1 , Xinfang Xu 1, 2
Affiliation
|
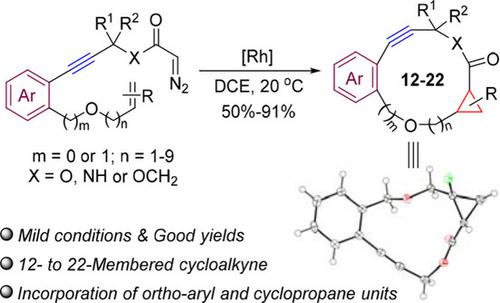
An efficient dirhodium(II)‐catalyzed macrocyclization reaction of alkyne‐containing diazoacetates through intramolecular metal carbene cyclopropanation is described. This method provides a variety of 12‐ to 22‐membered macrocyclic alkynes, which incorporate ortho‐aryl, cyclopropane, and cyclopropene units, in good to excellent yields under mild reaction conditions.
中文翻译:

吡啶(II)催化的含炔烃的α-重氮乙酸酯的环丙烷化反应,用于合成环炔烃
通过分子内金属卡宾环丙烷化反应,对含炔基的重氮乙酸酯进行了有效的由二氢吡啶鎓(II)催化的大环化反应。该方法可提供多种12至22元大环炔烃,这些炔烃结合了邻芳基,环丙烷和环丙烯单元,在温和的反应条件下收率良好。
更新日期:2020-08-04
中文翻译:

吡啶(II)催化的含炔烃的α-重氮乙酸酯的环丙烷化反应,用于合成环炔烃
通过分子内金属卡宾环丙烷化反应,对含炔基的重氮乙酸酯进行了有效的由二氢吡啶鎓(II)催化的大环化反应。该方法可提供多种12至22元大环炔烃,这些炔烃结合了邻芳基,环丙烷和环丙烯单元,在温和的反应条件下收率良好。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号