当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water Inhibition of Oxymethylene Dimethyl Ether Synthesis over Zeolite H-Beta: A Combined Kinetic and in Situ ATR-IR Study
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-22 , DOI: 10.1021/acscatal.0c01805
Christophe J. Baranowski 1 , Thibault Fovanna 1, 2 , Maneka Roger 1, 2 , Matteo Signorile 3 , Joseph McCaig 1 , Ali M. Bahmanpour 1 , Davide Ferri 2 , Oliver Kröcher 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-22 , DOI: 10.1021/acscatal.0c01805
Christophe J. Baranowski 1 , Thibault Fovanna 1, 2 , Maneka Roger 1, 2 , Matteo Signorile 3 , Joseph McCaig 1 , Ali M. Bahmanpour 1 , Davide Ferri 2 , Oliver Kröcher 1, 2
Affiliation
|
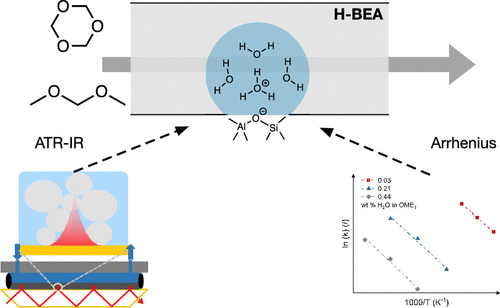
The effect of water on the kinetics of oxymethylene dimethyl ether (OME) synthesis from dimethoxymethane (OME1) and trioxane (TRI) has been investigated in a combined kinetic and in situ infrared spectroscopy study. The kinetic study revealed that a water content in OME1 as low as 0.21 wt % can significantly hamper the reaction rate. The apparent activation energy increased with the water concentration, but the frequency factor was more severely affected and decreased by an order of magnitude when the water concentration was doubled. With increasing water content, the chain growth mechanism shifted from competition between the direct insertion of TRI and the dissociation of TRI with formaldehyde incorporation, to reaction of TRI with water to form methylene glycol units which were inserted in the OME chain. The competition between water and the reactants for binding to the active sites of the zeolite was studied by means of modulated excitation attenuated total reflection infrared (ME-ATR-IR) spectroscopy experiments. It demonstrated a competition for silanol sites and Brønsted acid sites (BAS) according to the binding affinity order OME1 > H2O > TRI. This trend was confirmed by a DFT study of the interaction of OME1, TRI, and H2O with BAS. Combined together, these results indicated that the presence of water inhibited the adsorption of TRI on the binding sites, which prevented OME growth. Hence, even very low levels of water must be controlled for an efficient catalytic process.
中文翻译:

H-β沸石上甲醛对二甲醚合成的阻水作用:动力学和原位ATR-IR联合研究
在结合的动力学和原位红外光谱研究中,已经研究了水对由二甲氧基甲烷(OME 1)和三恶烷(TRI)合成甲醛二甲基醚(OME)动力学的影响。动力学研究表明,OME 1中的水分低至0.21wt%可显着阻碍反应速率。表观活化能随水的浓度增加而增加,但当水的浓度增加一倍时,频率因子受到的影响更大,并降低了一个数量级。随着水含量的增加,链增长的机理从直接插入TRI和引入甲醛的TRI离解之间的竞争转移到TRI与水反应形成亚甲基二醇单元的过程,该单元被插入OME链中。通过调制激发衰减全反射红外(ME-ATR-IR)光谱实验研究了水和反应物与沸石活性位点的结合竞争。1 > H 2 O> TRI。DOME研究OME 1,TRI和H 2 O与BAS的相互作用证实了这一趋势。综合起来,这些结果表明水的存在抑制了TRI在结合位点上的吸附,从而阻止了OME的生长。因此,为了有效的催化过程,甚至必须控制非常低的水含量。
更新日期:2020-08-08
中文翻译:

H-β沸石上甲醛对二甲醚合成的阻水作用:动力学和原位ATR-IR联合研究
在结合的动力学和原位红外光谱研究中,已经研究了水对由二甲氧基甲烷(OME 1)和三恶烷(TRI)合成甲醛二甲基醚(OME)动力学的影响。动力学研究表明,OME 1中的水分低至0.21wt%可显着阻碍反应速率。表观活化能随水的浓度增加而增加,但当水的浓度增加一倍时,频率因子受到的影响更大,并降低了一个数量级。随着水含量的增加,链增长的机理从直接插入TRI和引入甲醛的TRI离解之间的竞争转移到TRI与水反应形成亚甲基二醇单元的过程,该单元被插入OME链中。通过调制激发衰减全反射红外(ME-ATR-IR)光谱实验研究了水和反应物与沸石活性位点的结合竞争。1 > H 2 O> TRI。DOME研究OME 1,TRI和H 2 O与BAS的相互作用证实了这一趋势。综合起来,这些结果表明水的存在抑制了TRI在结合位点上的吸附,从而阻止了OME的生长。因此,为了有效的催化过程,甚至必须控制非常低的水含量。



































 京公网安备 11010802027423号
京公网安备 11010802027423号