European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-06-20 , DOI: 10.1016/j.ejmech.2020.112443 Sonia Martínez-González 1 , Ana Belén García 1 , M Isabel Albarrán 1 , Antonio Cebriá 1 , Adrián Amezquita-Alves 1 , Francisco Javier García-Campos 1 , Jaime Martínez-Gago 2 , Jorge Martínez-Torrecuadrada 2 , I G Muñoz 2 , Carmen Blanco-Aparicio 1 , Joaquín Pastor 1
|
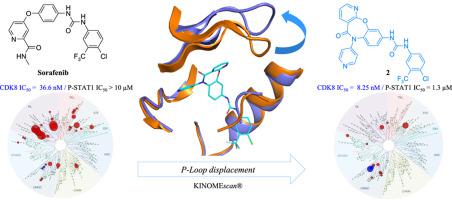
CDK8 is a cyclin-dependent kinase that forms part of the mediator complex, and modulates the transcriptional output from distinct transcription factors involved in oncogenic control. Overexpression of CDK8 has been observed in various cancers, representing a potential target for developing novel CDK8 inhibitors in cancer therapeutics. In the course of our investigations to discover new CDK8 inhibitors, we designed and synthesized tricyclic pyrido[2,3-b][1,5]benzoxazepin-5(6H)-one derivatives, by introduction of chemical complexity in the multi-kinase inhibitor Sorafenib taking into account the flexibility of the P-loop motif of CDK8 protein observed after analysis of structural information of co-crystallized CDK8 inhibitors. In vitro evaluation of the inhibitory activity of the prepared compounds against CDK8 led us to identify compound 2 as the most potent inhibitor of the series (IC50 = 8.25 nM). Co-crystal studies and the remarkable selectivity profile of compound 2 are presented. Compound 2 showed moderate reduction of phosphorylation of CDK8 substrate STAT1 in cells, in line with other reported Type II CDK8 inhibitors. We propose herein an alternative to find a potential therapeutic use for this chemical series.
中文翻译:

作为CDK8抑制剂的Pyrido [2,3-b] [1,5] benzoxazepin-5(6H)-one衍生物。
CDK8是细胞周期蛋白依赖性激酶,形成介质复合体的一部分,并调节来自与致癌控制有关的不同转录因子的转录输出。已经在各种癌症中观察到CDK8的过表达,代表了在癌症治疗剂中开发新型CDK8抑制剂的潜在目标。在探索新的CDK8抑制剂的研究过程中,我们通过在多激酶中引入化学复杂性,设计并合成了三环吡啶并[2,3-b] [1,5]苯并a嗪-5(6H)-one衍生物在分析共结晶CDK8抑制剂的结构信息后,考虑到了Sorafenib抑制剂Sorafenib的灵活性,考虑到了CDK8蛋白P环基序的灵活性。体外制备的化合物对CDK8的抑制活性的评估使我们确定化合物2是该系列中最有效的抑制剂(IC 50 = 8.25 nM)。介绍了共晶研究和化合物2的显着选择性。与其他报道的II型CDK8抑制剂相一致,化合物2在细胞中显示出CDK8底物STAT1磷酸化的适度降低。我们在此提出一种替代方案,以找到该化学系列的潜在治疗用途。































 京公网安备 11010802027423号
京公网安备 11010802027423号