当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Enhanced Electrocatalytic Hydrogen Evolution via Integrating Electrochemically Inactive g-C3N4: The Effect of Interfacial Engineering
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-06-19 , DOI: 10.1021/acssuschemeng.0c03938
Manqin Guan 1 , Chao Wang 1 , Shuo Li 2 , Haiwei Du 1, 3 , Yupeng Yuan 1, 3, 4
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-06-19 , DOI: 10.1021/acssuschemeng.0c03938
Manqin Guan 1 , Chao Wang 1 , Shuo Li 2 , Haiwei Du 1, 3 , Yupeng Yuan 1, 3, 4
Affiliation
|
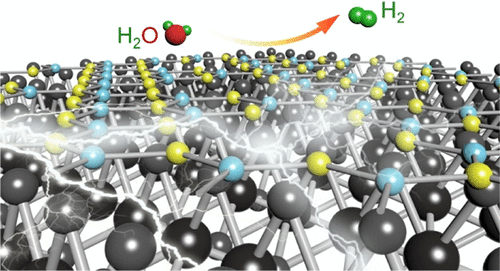
Electrocatalytic water splitting is a very promising hydrogen generation technology, and nonprecious electrocatalysts with outstanding performance are highly desired for future applications. Interfacial engineering, creating abundant interfaces with active sites when combining different materials, is of critical importance to not only modify the local structure of the electrocatalysts but also modulate the catalytic kinetics during the electrocatalytic reaction, thus offering a promising route toward unexpected electrocatalytic activity. Herein, graphitic carbon nitride (g-CN), which is electrochemically inert, is decorated onto the molybdenum disulphide (MoS2) surface to construct a MoS2/g-CN heterogeneous structure via a simple hydrothermal method. After integrating g-CN, an enlarged surface area, interfacial charge redistribution, and moderate surface-hydrogen adsorption are achieved without affecting the MoS2 morphology. Consequently, the MoS2/g-CN heterostructure exhibits a comparable electrocatalytic activity: a Tafel slope of 57 mV dec–1 and an overpotential of 141 mV at 10 mA cm–2. This work indicates the importance of interfacial engineering as an effective strategy for the rational design of electrocatalysts.
中文翻译:

通过集成电化学惰性gC 3 N 4了解增强的电催化氢逸出:界面工程的影响
电催化水分解是一种非常有前途的制氢技术,具有卓越性能的非贵金属电催化剂对于未来的应用是非常需要的。界面工程在组合不同的材料时可在活性位点上形成丰富的界面,这对修饰电催化剂的局部结构以及调节电催化反应过程中的催化动力学至关重要,因此为实现意想不到的电催化活性提供了有希望的途径。这里,石墨碳氮化物(G-CN),其是电化学惰性的,装饰到二硫化钼(MOS 2)表面来构造的MOS 2 /克- CN异质结构通过一种简单的水热法。集成g-CN之后,可以在不影响MoS 2形态的情况下实现更大的表面积,界面电荷再分布和适度的表面氢吸附。因此,MoS 2 / g-CN异质结构表现出可比的电催化活性:Tafel斜率为57 mV dec –1,在10 mA cm –2下的超电势为141 mV 。这项工作表明界面工程作为合理设计电催化剂的有效策略的重要性。
更新日期:2020-07-13
中文翻译:

通过集成电化学惰性gC 3 N 4了解增强的电催化氢逸出:界面工程的影响
电催化水分解是一种非常有前途的制氢技术,具有卓越性能的非贵金属电催化剂对于未来的应用是非常需要的。界面工程在组合不同的材料时可在活性位点上形成丰富的界面,这对修饰电催化剂的局部结构以及调节电催化反应过程中的催化动力学至关重要,因此为实现意想不到的电催化活性提供了有希望的途径。这里,石墨碳氮化物(G-CN),其是电化学惰性的,装饰到二硫化钼(MOS 2)表面来构造的MOS 2 /克- CN异质结构通过一种简单的水热法。集成g-CN之后,可以在不影响MoS 2形态的情况下实现更大的表面积,界面电荷再分布和适度的表面氢吸附。因此,MoS 2 / g-CN异质结构表现出可比的电催化活性:Tafel斜率为57 mV dec –1,在10 mA cm –2下的超电势为141 mV 。这项工作表明界面工程作为合理设计电催化剂的有效策略的重要性。







































 京公网安备 11010802027423号
京公网安备 11010802027423号