Science of the Total Environment ( IF 8.2 ) Pub Date : 2020-06-19 , DOI: 10.1016/j.scitotenv.2020.140213 Hongyan Zuo 1 , Ravi Kukkadapu 2 , Zihua Zhu 2 , Shuisong Ni 3 , Liuqin Huang 4 , Qiang Zeng 5 , Chongxuan Liu 6 , Hailiang Dong 7
|
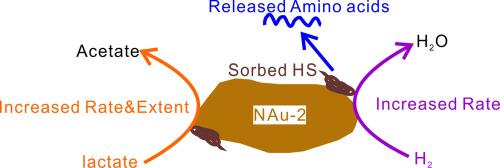
Previous studies have shown that humic substances can serve as electron shuttle to catalyze bioreduction of structural Fe(III) in clay minerals, but it is unclear if clay-sorbed humic substances can serve the same function. It is unknown if the electron shuttling function is dependent on electron donor type and if humic substances undergo change as a result. In this study, humic acid (HA) and fulvic acid (FA) were sorbed onto nontronite (NAu-2) surface. Structural Fe(III) in HA- and FA-coated NAu-2 samples was bioreduced by Shewanella putrefaciens CN32 using H2 and lactate as electron donors. The results showed a contrasting effect of humic substances on bioreduction of structural Fe(III), depending on the electron donor type. With H2 as electron donor, humic substances had little effect on bioreduction of Fe(III) (the reduction extent: 26.2%, 27.4%, 29.3% for HA-coated, FA-coated, and uncoated NAu-2, respectively). In contrast, these substances significantly enhanced bioreduction of Fe(III) with lactate as electron donor (the reduction extent: 20.2%, 20.7%, 11.5% for HA-coated, FA-coated, and uncoated NAu-2, respectively). This contrasting behavior is likely caused by the difference in reaction free energy and electron transport process between H2 and lactate. When H2 served as electron donor, more energy was released than when lactate served as electron donor. In addition, because of different cellular locations of lactate dehydrogenase (inner membrane) and H2 hydrogenase (the periplasm), electrons generated by H2 hydrogenase may pass through the electron transport chain more rapidly than those generated from lactate dehydrogenase. Through their functions as electron shuttle and/or carbon source, clay-sorbed HA/FA underwent partial transformation to amino acids and other compounds. The availability of external carbon source played an important role in the amount and type of secondary product generation. These results have important implications for coupled iron and carbon biogeochemical cycles in clay- and humic substance-rich environments.
中文翻译:

粘土相关腐殖质在腐殖酸希瓦氏菌CN32催化还原脱钙石中结构Fe(III)的生物还原中的作用。
先前的研究表明,腐殖质可以充当电子穿梭物,催化粘土矿物中结构性Fe(III)的生物还原,但是尚不清楚粘土吸附的腐殖质是否可以起到相同的作用。未知的是电子穿梭功能是否取决于电子给体的类型以及腐殖质是否随之发生变化。在这项研究中,腐殖酸(HA)和黄腐酸(FA)被吸附到囊脱石(NAu-2)表面。结构的Fe(III)在HA-和FA-涂覆NAU-2样品被生物还原腐败希瓦氏菌CN32使用H 2和乳酸作为电子供体。结果表明,取决于电子给体的类型,腐殖质对结构性Fe(III)生物还原的反差作用。与H 2作为电子供体,腐殖质对Fe(III)的生物还原影响很小(HA涂层,FA涂层和未涂层的NAu-2的还原程度分别为26.2%,27.4%,29.3%)。相比之下,这些物质显着增强了以乳酸作为电子供体的Fe(III)的生物还原作用(HA涂层,FA涂层和未涂层的NAu-2的还原程度分别为20.2%,20.7%,11.5%)。这种相反的行为可能是由H 2和乳酸之间的反应自由能和电子传输过程的差异引起的。当H 2用作电子给体时,释放出的能量比乳酸作为电子给体时释放的能量更多。另外,由于乳酸脱氢酶(内膜)和H 2的细胞位置不同氢酶(周质),由H 2氢化酶产生的电子可能比从乳酸脱氢酶产生的电子更快地通过电子传输链。通过其作为电子穿梭和/或碳源的功能,粘土吸附的HA / FA经历了部分转化为氨基酸和其他化合物的作用。外部碳源的可利用性在次级产品生成的数量和类型中起着重要作用。这些结果对于在富含粘土和腐殖质的环境中的铁和碳生物地球化学循环具有重要意义。

































 京公网安备 11010802027423号
京公网安备 11010802027423号