当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unravelling the Role of Structural Geometry and Chemical State of Well-Defined Oxygen Vacancies on Pristine CeO2 for H2O2 Activation.
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.jpclett.0c01557 Zicong Tan,Jieru Zhang,Yu-Cheng Chen,Jyh-Pin Chou,Yung-Kang Peng
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2020-06-17 , DOI: 10.1021/acs.jpclett.0c01557 Zicong Tan,Jieru Zhang,Yu-Cheng Chen,Jyh-Pin Chou,Yung-Kang Peng
|
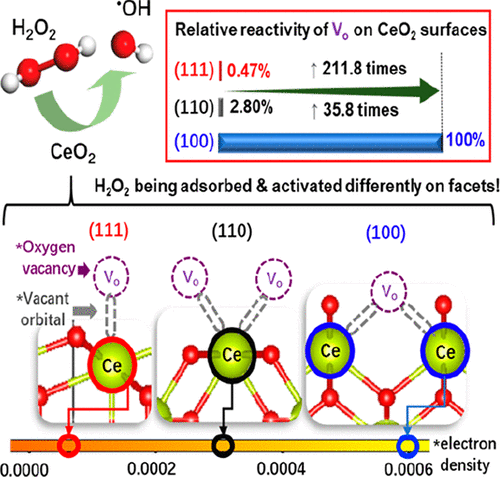
Although H2O2 has been often employed as a green oxidant for many CeO2-catalyzed reactions, the underlying principle of its activation by surface oxygen vacancy (Vo) is still elusive due to the irreversible removal of postgenerated Vo by water (or H2O2). The metastable Vo (ms-Vo) naturally preserved on pristine CeO2 surfaces was adopted herein for an in-depth study of their interplay with H2O2. Their well-defined local structures and chemical states were found facet-dependent affecting both the adsorption and subsequent activation of H2O2. It is concluded that a strong adsorption of H2O2 on ms-Vo may not guarantee its subsequent activation. The ms-Vo can be only free for the next catalytic cycle when the electron density of surface Ce is high enough to reduce/break the O–O bond of adsorbed H2O2. This explains the 211.8 and 35.8 times enhancement in H2O2 reactivity when the CeO2 surface is changed from (111) and (110) to (100).
中文翻译:

阐明结构良好的氧空位的结构几何形状和化学状态在原始CeO2上对H2O2活化的作用。
尽管H 2 O 2在许多CeO 2催化的反应中经常被用作绿色氧化剂,但由于水不可逆地去除后生成的V o,其通过表面氧空位(V o)活化的基本原理仍然难以捉摸(或H 2 O 2)。本文采用天然保留在原始CeO 2表面上的亚稳态V o(ms-V o)来深入研究它们与H 2 O 2的相互作用。发现它们的明确定义的局部结构和化学状态取决于刻面,从而影响H 2 O 2的吸附和随后的活化。结论是H 2 O 2在ms-V o上的强吸附可能无法保证其随后的活化。当表面Ce的电子密度高到足以降低/破坏吸附的H 2 O 2的O-O键时,ms-V o才可以在下一个催化循环中释放。这解释了当CeO 2表面从(111)和(110)变为(100)时,H 2 O 2反应性提高了211.8和35.8倍。
更新日期:2020-07-16
中文翻译:

阐明结构良好的氧空位的结构几何形状和化学状态在原始CeO2上对H2O2活化的作用。
尽管H 2 O 2在许多CeO 2催化的反应中经常被用作绿色氧化剂,但由于水不可逆地去除后生成的V o,其通过表面氧空位(V o)活化的基本原理仍然难以捉摸(或H 2 O 2)。本文采用天然保留在原始CeO 2表面上的亚稳态V o(ms-V o)来深入研究它们与H 2 O 2的相互作用。发现它们的明确定义的局部结构和化学状态取决于刻面,从而影响H 2 O 2的吸附和随后的活化。结论是H 2 O 2在ms-V o上的强吸附可能无法保证其随后的活化。当表面Ce的电子密度高到足以降低/破坏吸附的H 2 O 2的O-O键时,ms-V o才可以在下一个催化循环中释放。这解释了当CeO 2表面从(111)和(110)变为(100)时,H 2 O 2反应性提高了211.8和35.8倍。












































 京公网安备 11010802027423号
京公网安备 11010802027423号