Electrochimica Acta ( IF 5.5 ) Pub Date : 2020-06-17 , DOI: 10.1016/j.electacta.2020.136625 Aline R. Dória , Ronaldo S. Silva , Paulo H. Oliveira Júnior , Euler A. dos Santos , Silvana Mattedi , Peter Hammer , Giancarlo R. Salazar-Banda , Katlin I.B. Eguiluz
|
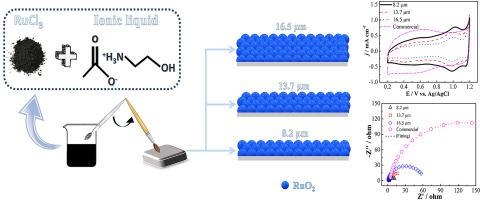
The use of ionic liquids as solvents to prepare mixed metal oxide (MMO) anodes has proven to be a very promising alternative synthesis route. However, little attention has been dedicated to assessing the effect of the coating thickness on their activity and service life, and no detailed study of the thickness of anodes made using ionic liquids has been conducted to date. Here we studied the influence of the ruthenium oxides film thickness (8.2, 13.7, and 16.5 μm) on the physical, electrochemical, and electrocatalytic properties of Ti/RuO2 anodes. The electrodes were prepared using the 2-hydroxyethyl ammonium acetate (2HEAA) ionic liquid as the solvent of the precursor solutions. The properties of these anodes were compared with the RuO2-based commercial anode provided by De Nora® (17.7 μm of thickness). The increase in RuO2 layer thickness from 8.2 μm to 13.7 μm, and then to 16.5 μm decreases the voltammetric anode charge and increases their charge transfer resistance from 11.2 Ω to 28.2 Ω, and then to 52.7 Ω, respectively. Deactivation of the anodes was investigated by accelerated lifetime tests in H2SO4 and NaCl solutions. In acidic media, the anodes with a higher thickness (13.7 μm and 16.5 μm) presented longer service lifetimes, respectively, 1.5 and 1.4 higher than the electrode with 8.2 μm. In NaCl medium, a higher thickness improves the service life significantly. The anode with 16.5 μm of thickness has a service life up to 48.6 times higher than the anode with 8.2 μm. All anodes, including the commercial one, presented high catalytic efficiency towards the electrochemical degradation of Reactive Black 5 dye. Within only 30 min of electrochemical treatment, all anodes removed more than 94% of color. It is shown that the use of anodes with low coating thicknesses is more favorable due to reduced time and energy costs of the synthesis process while maintaining a high degradation efficiency of the dye.
中文翻译:

RuO 2层厚度对离子液体法合成阳极的物理和电化学性能的影响
已证明使用离子液体作为溶剂来制备混合金属氧化物(MMO)阳极是一种非常有前途的替代合成途径。然而,很少有人致力于评估涂层厚度对其活性和使用寿命的影响,并且迄今为止,尚未对使用离子液体制成的阳极厚度进行详细的研究。在这里,我们研究了钌氧化物膜厚度(8.2、13.7和16.5μm)对Ti / RuO 2阳极的物理,电化学和电催化性能的影响。使用乙酸2-羟乙基铵(2HEAA)离子液体作为前体溶液的溶剂制备电极。将这些阳极的性质与RuO 2进行了比较DeNora®提供的基于商业的阳极(厚度为17.7μm)。RuO 2层厚度从8.2μm增加到13.7μm,然后增加到16.5μm,会减少伏安阳极电荷,并将其电荷转移电阻分别从11.2Ω增加到28.2Ω,然后增加到52.7Ω。通过在H 2 SO 4中进行加速寿命试验研究了阳极的失活和氯化钠溶液。在酸性介质中,具有较高厚度(13.7μm和16.5μm)的阳极分别具有更长的使用寿命,分别比具有8.2μm的电极高1.5和1.4倍。在NaCl介质中,较高的厚度可以显着提高使用寿命。厚度为16.5μm的阳极使用寿命是8.2μm阳极的48.6倍。所有阳极,包括商用阳极,都对活性黑5染料的电化学降解表现出很高的催化效率。在仅30分钟的电化学处理过程中,所有阳极去除的颜色超过94%。结果表明,由于减少了合成过程的时间和能量成本,同时保持了染料的高降解效率,使用具有低涂层厚度的阳极是更有利的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号