当前位置:
X-MOL 学术
›
Int. J. Food Sci. Tech.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of natural trypsin inhibitor from soybean on texture deterioration of the bay scallop (Argopecten irradians) during cold storage and its mechanism
International Journal of Food Science & Technology ( IF 2.6 ) Pub Date : 2020-06-15 , DOI: 10.1111/ijfs.14676 Bing Liu 1, 2 , Yu‐xin Liu 1, 2 , Zi‐qiang Liu 1 , Man‐man Yu 1 , Hui‐lin Liu 1, 2 , Lei Qin 1, 2 , Da‐yong Zhou 1, 2, 3 , Bei‐wei Zhu 1, 2, 3
International Journal of Food Science & Technology ( IF 2.6 ) Pub Date : 2020-06-15 , DOI: 10.1111/ijfs.14676 Bing Liu 1, 2 , Yu‐xin Liu 1, 2 , Zi‐qiang Liu 1 , Man‐man Yu 1 , Hui‐lin Liu 1, 2 , Lei Qin 1, 2 , Da‐yong Zhou 1, 2, 3 , Bei‐wei Zhu 1, 2, 3
Affiliation
|
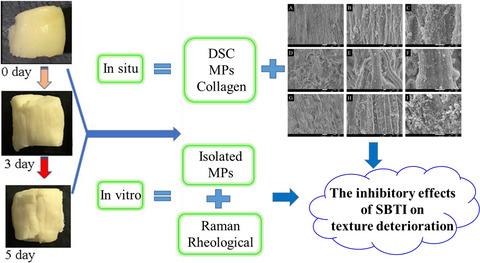
In this study, the inhibitory effects of natural trypsin inhibitor from soybean (SBTI) on texture deterioration of adductor muscle of scallop (AMS) during cold storage and its mechanism were revealed by determining the changes of structural proteins of AMS in situ and in vitro, respectively. Results indicated that the endogenous proteolysis resulted in the disintegration of connective tissue proteins, the degradation of myosin and actin, as well as the reduction of α‐helix, the increase of β‐sheet and amino acid side chains, and the exposure of tryptophan residues of isolated myofibrillar proteins (MPs). These changes in structural proteins contribute to the texture deterioration of AMS as reflected in a significant decrease in hardness, chewiness, adhesiveness and springiness, but a significant increase in cohesiveness. SBTI significantly inhibited the conformational changes of structural proteins caused by endogenous proteolysis, improved the MP gel‐forming ability and consequently retarded the texture deterioration of AMS.
中文翻译:

大豆天然胰蛋白酶抑制剂对冷藏期间海湾扇贝(Argopecten irradians)质地退化的影响及其机理
通过测定原位和体外AMS结构蛋白的变化,揭示了大豆天然胰蛋白酶抑制剂(SBTI)对扇贝(AMS)内收肌纹理退化的抑制作用及其机理。, 分别。结果表明,内源性蛋白水解导致结缔组织蛋白的分解,肌球蛋白和肌动蛋白的降解,以及α-螺旋的减少,β-折叠和氨基酸侧链的增加以及色氨酸残基的暴露分离的肌原纤维蛋白(MPs)。结构蛋白的这些变化导致AMS的质地下降,这反映为硬度,咀嚼性,粘合性和弹性的显着降低,但内聚力的显着提高。SBTI显着抑制了内源性蛋白水解引起的结构蛋白的构象变化,提高了MP凝胶形成能力,从而抑制了AMS的质地劣化。
更新日期:2020-06-15
中文翻译:

大豆天然胰蛋白酶抑制剂对冷藏期间海湾扇贝(Argopecten irradians)质地退化的影响及其机理
通过测定原位和体外AMS结构蛋白的变化,揭示了大豆天然胰蛋白酶抑制剂(SBTI)对扇贝(AMS)内收肌纹理退化的抑制作用及其机理。, 分别。结果表明,内源性蛋白水解导致结缔组织蛋白的分解,肌球蛋白和肌动蛋白的降解,以及α-螺旋的减少,β-折叠和氨基酸侧链的增加以及色氨酸残基的暴露分离的肌原纤维蛋白(MPs)。结构蛋白的这些变化导致AMS的质地下降,这反映为硬度,咀嚼性,粘合性和弹性的显着降低,但内聚力的显着提高。SBTI显着抑制了内源性蛋白水解引起的结构蛋白的构象变化,提高了MP凝胶形成能力,从而抑制了AMS的质地劣化。































 京公网安备 11010802027423号
京公网安备 11010802027423号