当前位置:
X-MOL 学术
›
J. Cell. Physiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Paeoniflorin promotes angiogenesis and tissue regeneration in a full-thickness cutaneous wound model through the PI3K/AKT pathway.
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-06-15 , DOI: 10.1002/jcp.29808 Xiaoyu Dong 1, 2 , Zili He 1, 2 , Guangheng Xiang 1, 2 , Leyi Cai 1, 2 , Zhenjiang Xu 1, 2 , Cong Mao 1, 2 , Yongzeng Feng 1, 2
Journal of Cellular Physiology ( IF 4.5 ) Pub Date : 2020-06-15 , DOI: 10.1002/jcp.29808 Xiaoyu Dong 1, 2 , Zili He 1, 2 , Guangheng Xiang 1, 2 , Leyi Cai 1, 2 , Zhenjiang Xu 1, 2 , Cong Mao 1, 2 , Yongzeng Feng 1, 2
Affiliation
|
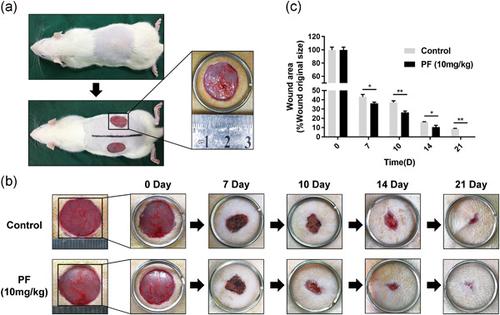
The treatment of wounds remains a clinical challenge because of poor angiogenesis under the wound bed, and increasingly, the patients' need for functional and aesthetically pleasing scars. For the wound healing process, new blood vessels which can deliver nutrients and oxygen to the wound area are necessary. In this study, we investigated the pro‐angiogenesis ability and mechanism in wound healing of paeoniflorin (PF), which is a traditional Chinese medicine. In our in vitro results, the ability for proliferation, migration and in vitro angiogenesis in human umbilical vein endothelial cells was promoted by coculturing with PF (1.25–5 μM). Meanwhile, molecular docking studies revealed that PF has excellent binding abilities to phosphatidylinositol‐3‐kinase (PI3K) and protein kinase B (AKT), and consistent with our western blot results, that PF suppressed PI3K and AKT phosphorylation. Furthermore, to investigate the healing effect of PF in vivo, we constructed a full‐thickness cutaneous wound model in rats. PF stimulated the cellular proliferation status, collagen matrix deposition and remodeling processes in vitro and new blood vessel formation at the wound bed resulting in efficient wound healing after intragastric administration of 10 mg·kg−1·day−1 in vivo. Overall, PF performed the pro‐angiogenetic effect in vitro and accelerating wound healing in vivo. In summary, the capacity for angiogenesis in endothelial cells could be enhanced by PF treatment via the PI3K/AKT pathway in vitro and could accelerate the wound healing process in vivo through collagen deposition and angiogenesis in regenerated tissue. This study provides evidence that application of PF represents a novel therapeutic approach for the treatment of cutaneous wounds.
中文翻译:

芍药苷通过 PI3K/AKT 通路促进全层皮肤伤口模型中的血管生成和组织再生。
由于伤口床下血管生成不良,以及患者对功能性和美观性疤痕的需求日益增加,伤口的治疗仍然是一个临床挑战。对于伤口愈合过程,可以将营养和氧气输送到伤口区域的新血管是必要的。在这项研究中,我们研究了中药芍药苷 (PF) 在伤口愈合中的促血管生成能力和机制。在我们的体外结果中,通过与 PF (1.25-5 μM) 共培养促进了人脐静脉内皮细胞的增殖、迁移和体外血管生成能力。同时,分子对接研究表明,PF对磷脂酰肌醇-3-激酶(PI3K)和蛋白激酶B(AKT)具有优异的结合能力,与我们的蛋白质印迹结果一致,PF 抑制 PI3K 和 AKT 磷酸化。此外,为了研究 PF 在体内的愈合效果,我们构建了大鼠全层皮肤伤口模型。PF 刺激体外细胞增殖状态、胶原基质沉积和重塑过程以及伤口床新血管形成,从而在灌胃 10 mg·kg 后导致伤口有效愈合-1 ·day -1体内。总体而言,PF 在体外具有促血管生成作用并在体内加速伤口愈合。总之,PF 治疗可通过体外 PI3K/AKT 通路增强内皮细胞的血管生成能力,并可通过再生组织中的胶原沉积和血管生成加速体内伤口愈合过程。这项研究提供的证据表明,PF 的应用代表了一种治疗皮肤伤口的新治疗方法。
更新日期:2020-06-15
中文翻译:

芍药苷通过 PI3K/AKT 通路促进全层皮肤伤口模型中的血管生成和组织再生。
由于伤口床下血管生成不良,以及患者对功能性和美观性疤痕的需求日益增加,伤口的治疗仍然是一个临床挑战。对于伤口愈合过程,可以将营养和氧气输送到伤口区域的新血管是必要的。在这项研究中,我们研究了中药芍药苷 (PF) 在伤口愈合中的促血管生成能力和机制。在我们的体外结果中,通过与 PF (1.25-5 μM) 共培养促进了人脐静脉内皮细胞的增殖、迁移和体外血管生成能力。同时,分子对接研究表明,PF对磷脂酰肌醇-3-激酶(PI3K)和蛋白激酶B(AKT)具有优异的结合能力,与我们的蛋白质印迹结果一致,PF 抑制 PI3K 和 AKT 磷酸化。此外,为了研究 PF 在体内的愈合效果,我们构建了大鼠全层皮肤伤口模型。PF 刺激体外细胞增殖状态、胶原基质沉积和重塑过程以及伤口床新血管形成,从而在灌胃 10 mg·kg 后导致伤口有效愈合-1 ·day -1体内。总体而言,PF 在体外具有促血管生成作用并在体内加速伤口愈合。总之,PF 治疗可通过体外 PI3K/AKT 通路增强内皮细胞的血管生成能力,并可通过再生组织中的胶原沉积和血管生成加速体内伤口愈合过程。这项研究提供的证据表明,PF 的应用代表了一种治疗皮肤伤口的新治疗方法。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号