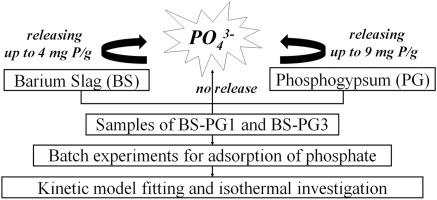
钡渣(BS)是钡盐工业流程中的废渣。由于其Ba 2+的高浸出浓度,BS被归类为一种危险废物。由于所含的难溶硫酸盐,工业废磷石膏(PG)可有效地将钡离子固定在BS中。在这项研究中,选择了两种不同比例的PG与BS混合以固化可溶性钡离子。然后将经过一定比例的PG(BS-PG1,BS-PG3)处理的无害BS样品功能性地用于溶液中的磷酸盐去除。进行了批处理实验以去除磷酸盐,以评估BS-PG1和BS-PG3的吸附效率。研究了接触时间,初始pH和反应温度等各种因素对吸附性能的影响。初始浓度为15 mg / L时,BS-PG1和BS-PG3在约3小时内达到吸附平衡,BS-PG1的吸附容量为12.47 mg P / g,在固体:液体,1g:1L,25°C,自然pH的条件下高于BS(11.49 mg P / g)。结果表明,磷酸根离子在BS-PG1和BS-PG3上的吸附过程均符合拟二级动力学模型。朗格缪尔等温模型被认为是实验数据的合适方程,显示了对磷酸盐的最大吸附能力,分别达到BS-PG1和BS-PG3的13.67 mg P / g和11.59 mg P / g。与其他吸附剂相比,BS-PG1和BS-PG3被认为是去除磷酸盐的有效材料。朗格缪尔等温模型被认为是实验数据的合适方程,显示了对磷酸盐的最大吸附能力,分别达到BS-PG1和BS-PG3的13.67 mg P / g和11.59 mg P / g。与其他吸附剂相比,BS-PG1和BS-PG3被认为是去除磷酸盐的有效材料。朗格缪尔等温模型被认为是实验数据的合适方程,显示了对磷酸盐的最大吸附能力,分别达到BS-PG1和BS-PG3的13.67 mg P / g和11.59 mg P / g。与其他吸附剂相比,BS-PG1和BS-PG3被认为是去除磷酸盐的有效材料。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Increasing phosphate sorption on barium slag by adding phosphogypsum for non-hazardous treatment.
Barium slag (BS) is a waste residue in the barium salt industrial procedure. Due to its high leaching concentration of Ba2+, BS is classified as a kind of hazardous waste. Industrial waste phosphogypsum (PG) is effective to immobilize barium ion in BS owing to the slightly soluble sulfate included. In this study, two different proportions of PG were selected for mixing with BS to solidify soluble barium ion. The non-hazardous BS samples treated with the proportions of PG (BS-PG1, BS-PG3) were then functionally used for phosphate removal in solution. Batch experiments for removal of phosphate were performed to evaluate the adsorption efficiency of BS-PG1 and BS-PG3. The effect of various factors such as contact time, initial pH, and reaction temperature on sorption performance was investigated. BS-PG1 and BS-PG3 reached adsorption equilibrium in approximately 3h at the initial concentration of 15 mg/L, and BS-PG1 exhibited adsorption capacity of 12.47 mg P/g, higher than that of BS (11.49 mg P/g) under the condition of solid:liquid, 1g:1L, 25 °C, natural pH. The results show that the adsorption processes of phosphates ions onto both BS-PG1 and BS-PG3 fitted well with the pseudo-second-order kinetic model. The Langmuir isothermal model was considered as the appropriate equation for experimental data, showing a maximum adsorption capacity for phosphate up to 13.67 mg P/g and 11.59 mg P/g for BS-PG1 and BS-PG3. In comparison with other adsorbents, BS-PG1 and BS-PG3 could be considered as efficient materials for the removal of phosphate.


































 京公网安备 11010802027423号
京公网安备 11010802027423号