Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-06-13 , DOI: 10.1016/j.cej.2020.125881 Xiaowei Sun , Dongyan Xu , Ping Dai , Xien Liu , Feng Tan , Qingjie Guo
|
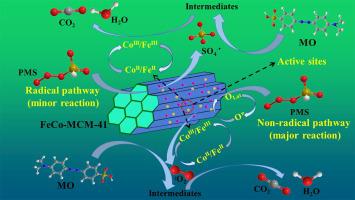
Cobalt-mediated activation of peroxymonosulfate (PMS) has been widely investigated for the effective oxidation of organic contaminants in wastewater. Herein, monometal- and bimetal-doped MCM-41 catalysts (Fe-MCM-41, Co-MCM-41, and FeCo-MCM-41) were synthesized by using one-pot hydrothermal method and attempted to degrade artificial methyl orange (MO) dye wastewater via PMS activation. The influences of initial PMS concentration, pH, catalyst dosage and reaction temperature on the degradation efficiency of MO were systematically examined. Compared with the contrasting catalysts, FeCo-MCM-41 exhibited extremely higher activity and lower amount of metal leaching in the degradation process. The excellent catalytic activity of FeCo-MCM-41 was ascribed to the high dispersion of metals and the synergistic effects of Co2+/Co3+ and Fe2+/Fe3+ redox cycles. A series of radical inhibition and electron paramagnetic resonance experiments revealed that both radical and non-radical pathways were involved in the degradation of MO. Singlet oxygen (1O2) was unveiled to be the dominant reactive oxygen species in the FeCo-MCM-41/PMS system. The possible degradation pathways were proposed based on the identification of intermediate products generated in the degradation process by LC-MS analyses.
中文翻译:

使用Fe-Co双金属掺杂的MCM-41作为过氧单硫酸盐活化剂通过自由基和非自由基途径有效降解水中的甲基橙
钴介导的过氧单硫酸盐(PMS)活化已被广泛研究以有效氧化废水中的有机污染物。在此,通过单锅水热法合成了单金属和双金属掺杂的MCM-41催化剂(Fe-MCM-41,Co-MCM-41和FeCo-MCM-41),并尝试降解人造甲基橙(MO )通过PMS活化处理染料废水。系统地研究了初始PMS浓度,pH,催化剂用量和反应温度对MO降解效率的影响。与对比催化剂相比,FeCo-MCM-41在降解过程中表现出极高的活性和较低的金属浸出量。FeCo-MCM-41的优异催化活性归因于金属的高分散性和Co 2+的协同作用/ Co 3+和Fe 2+ / Fe 3+的氧化还原循环。一系列的自由基抑制和电子顺磁共振实验表明,自由基和非自由基途径均与MO的降解有关。单重态氧(1 O 2)被揭示为FeCo-MCM-41 / PMS系统中的主要活性氧。在通过LC-MS分析鉴定降解过程中产生的中间产物的基础上,提出了可能的降解途径。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号