Molecular Catalysis ( IF 3.9 ) Pub Date : 2020-06-12 , DOI: 10.1016/j.mcat.2020.111054 Jilong Xue , Ying Wang , Yue Meng , Xiaobo Zhou , Guoxiang Pan , Shengjie Xia
|
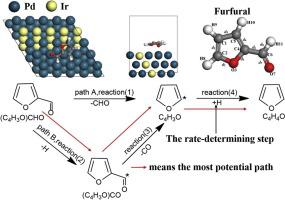
In this paper, decarbonylation of furfural on the surface of Pd(111) and M/Pd(111) (M=Ru,Ni,Ir) has been studied by DFT calculation. The adsorption energy, activation energy and reaction energy of decarbonylation of furfural on different metal surface were calculated and compared. The decarbonylation mechanism of furfural on the surfaces of Pd(111), Ni/Pd(111), Ru/Pd(111) and Ir/Pd(111) was discussed from two reaction routes and four elementary reactions. It is found that the energy of C4H3O*+H*→C4H4O is the highest, which is the rate determining step. The difference between the two reaction paths of the four catalysts shows that bimetallic catalyst can lower the activation energy of each reaction step more than single metal catalyst. Compared with the other three metals, on Ir/Pd(111) surface, all the reactions are exothermic with low activation energy. It is suggested that Ir/Pd(111) may be the best catalyst for decarbonylation of furfural.
中文翻译:

糠醛在Pd(111)和M / Pd(111)(M = Ru,Ni,Ir)表面上脱羰机理的理论研究
本文通过DFT计算研究了Pd(111)和M / Pd(111)(M = Ru,Ni,Ir)上糠醛的脱羰作用。计算并比较了糠醛在不同金属表面上的吸附能,活化能和反应能。从两个反应路线和四个基本反应出发,探讨了糠醛在Pd(111),Ni / Pd(111),Ru / Pd(111)和Ir / Pd(111)表面的脱羰机理。发现C 4 H 3 O * + H *→C 4 H 4的能量O是最高的,这是速率确定步骤。四种催化剂的两条反应路径之间的差异表明,双金属催化剂比单金属催化剂能更有效地降低每个反应步骤的活化能。与其他三种金属相比,在Ir / Pd(111)表面上,所有反应都是放热反应,活化能低。提示Ir / Pd(111)可能是糠醛脱羰反应的最佳催化剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号