当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Switching the Mallory Reaction to Synthesis of Naphthalenes, Benzannulated Heterocycles, and Their Derivatives.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-12 , DOI: 10.1021/acs.joc.0c00924 Andrey G Lvov 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-12 , DOI: 10.1021/acs.joc.0c00924 Andrey G Lvov 1, 2
Affiliation
|
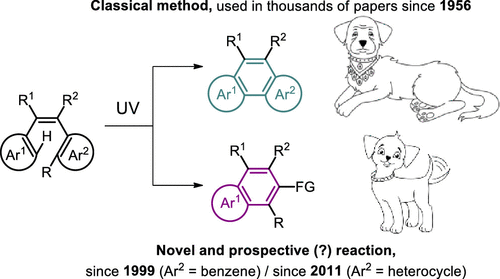
This review analyzes the new life of a long-known reaction, the photocyclization of diarylethenes, which became a classical tool for the synthesis of phenanthrenes and their heterocyclic analogues (Mallory reaction). It has been shown in recent years that certain diarylethenes undergo photorearrangement to naphthalenes, benzannulated heterocycles, or related products with bicyclic unit. Herein, I analyze how the Mallory reaction path can be altered to obtain bicyclic rather than tricyclic products. The mechanistic aspects and scope of the reaction are discussed.
中文翻译:

将马洛反应转换为萘,苯环化的杂环及其衍生物的合成。
这篇综述分析了一个众所周知的反应的新生命,即二芳烃的光环化,该反应成为合成菲及其杂环类似物(马洛反应)的经典工具。近年来已显示某些二芳烃经历光重排成萘,苯环二杂环或具有双环单元的相关产物。在这里,我分析了如何改变马洛反应路径以获得双环而不是三环产物。讨论了反应的机理和范围。
更新日期:2020-07-17
中文翻译:

将马洛反应转换为萘,苯环化的杂环及其衍生物的合成。
这篇综述分析了一个众所周知的反应的新生命,即二芳烃的光环化,该反应成为合成菲及其杂环类似物(马洛反应)的经典工具。近年来已显示某些二芳烃经历光重排成萘,苯环二杂环或具有双环单元的相关产物。在这里,我分析了如何改变马洛反应路径以获得双环而不是三环产物。讨论了反应的机理和范围。





























 京公网安备 11010802027423号
京公网安备 11010802027423号