当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
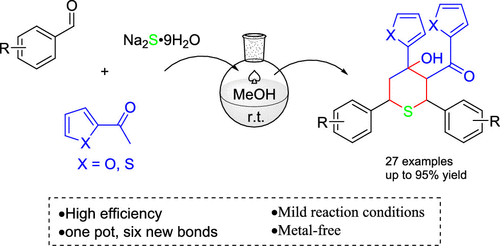
Domino Synthetic Strategy for Tetrahydrothiopyran Derivatives from Benzaldehydes, 2-Acetylfuran/2-Acetylthiophene, and Sodium Sulfide.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-12 , DOI: 10.1021/acs.joc.0c01006 Dongdong Chen 1 , Weixia Du 1 , Xufeng Yang 1 , Tao Liu 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-12 , DOI: 10.1021/acs.joc.0c01006 Dongdong Chen 1 , Weixia Du 1 , Xufeng Yang 1 , Tao Liu 1
Affiliation
|
A novel domino reaction from benzaldehydes and 2-acetylfuran/2-acetylthiophene with sodium sulfide was developed to synthesize a series of tetrahydrothiopyran (THTP) derivatives. The reaction proceeded well to construct a tetrahydrothiopyran ring and five new bonds in one step. A mechanism is proposed, involving a stepwise Aldol/double Michael addition/Aldol (AMMA) reaction cascade. In this transformation, sodium sulfide acts as a nucleophile and base. This method is characterized by transition-metal-free, commercially available starting materials and mild reaction conditions.
中文翻译:

苯甲醛,2-乙酰呋喃/ 2-乙酰噻吩和硫化钠的四氢噻喃衍生物的多米诺合成策略。
建立了由苯甲醛和2-乙酰基呋喃/ 2-乙酰基噻吩与硫化钠形成的新型多米诺反应,以合成一系列四氢噻喃(THTP)衍生物。反应进行得很好,一步就可以构建一个四氢噻喃环和五个新的键。提出了一种涉及逐步Aldol /双迈克尔加成/ Aldol(AMMA)反应级联的机理。在该转化中,硫化钠充当亲核试剂和碱。该方法的特征在于无过渡金属的市售起始原料和温和的反应条件。
更新日期:2020-07-17
中文翻译:

苯甲醛,2-乙酰呋喃/ 2-乙酰噻吩和硫化钠的四氢噻喃衍生物的多米诺合成策略。
建立了由苯甲醛和2-乙酰基呋喃/ 2-乙酰基噻吩与硫化钠形成的新型多米诺反应,以合成一系列四氢噻喃(THTP)衍生物。反应进行得很好,一步就可以构建一个四氢噻喃环和五个新的键。提出了一种涉及逐步Aldol /双迈克尔加成/ Aldol(AMMA)反应级联的机理。在该转化中,硫化钠充当亲核试剂和碱。该方法的特征在于无过渡金属的市售起始原料和温和的反应条件。








































 京公网安备 11010802027423号
京公网安备 11010802027423号