当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Strategy for Hydrazine Synthesis: Development and Overpotential Analysis of Methods for Oxidative N–N Coupling of an Ammonia Surrogate
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-10 , DOI: 10.1021/jacs.0c04626 Fei Wang 1 , James B Gerken 1 , Desiree M Bates 1 , Yeon Jung Kim 1 , Shannon S Stahl 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-10 , DOI: 10.1021/jacs.0c04626 Fei Wang 1 , James B Gerken 1 , Desiree M Bates 1 , Yeon Jung Kim 1 , Shannon S Stahl 1
Affiliation
|
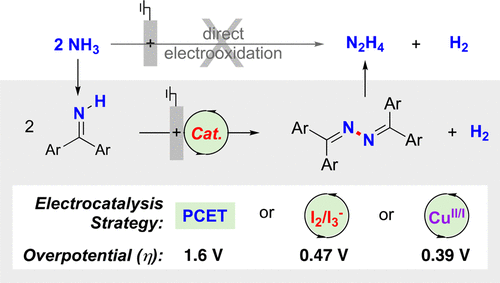
Hydrazine is an important industrial chemical and fuel that attracted considerable attention for use in liquid fuel cells. Ideally, hydrazine could be prepared via direct oxidative coupling of ammonia, but thermodynamic and kinetic factors limit the viability of this approach. The present study evaluates three different electrochemical strategies for the oxidative homocoupling of benzophenone imine, a readily accessible ammonia surrogate. Hydrolysis of the resulting benzophenone azine affords hydrazine and benzophenone, with the latter amenable to recycling. The three different electrochemical N-N coupling methods include (1) a proton-coupled electron-transfer process promoted by a phosphate base, (2) an iodine-mediated reaction involving intermediate N-I bond formation, and (3) a copper-catalyzed N-N coupling process. Analysis of the thermodynamic efficiencies for these electrochemical imine-to-azine oxidation reactions reveals low overpotentials (η) for the copper and iodine mediated processes (390 and 470 mV, respectively), but a much higher value for the proton-coupled pathway (η ~ 1.6 V). A similar approach is used to assess molecular electrocatalytic methods for electrochemical oxidation of ammonia to dinitrogen.
中文翻译:

肼合成的电化学策略:氨替代物氧化 N-N 偶联方法的开发和过电势分析
肼是一种重要的工业化学品和燃料,在液体燃料电池中的应用引起了广泛的关注。理想情况下,肼可以通过氨的直接氧化偶联来制备,但热力学和动力学因素限制了这种方法的可行性。本研究评估了二苯甲酮亚胺(一种易于获得的氨替代物)氧化自偶联的三种不同电化学策略。所得二苯甲酮吖嗪水解得到肼和二苯甲酮,后者可回收。三种不同的电化学神经网络耦合方法包括(1)由磷酸盐碱促进的质子耦合电子转移过程,(2)涉及中间NI键形成的碘介导反应,以及(3)铜催化的神经网络耦合过程。对这些电化学亚胺到吖嗪氧化反应的热力学效率的分析表明,铜和碘介导的过程具有较低的过电势 (η)(分别为 390 和 470 mV),但质子耦合途径的过电势值要高得多(η) 〜1.6V)。类似的方法用于评估将氨电化学氧化为氮气的分子电催化方法。
更新日期:2020-06-10
中文翻译:

肼合成的电化学策略:氨替代物氧化 N-N 偶联方法的开发和过电势分析
肼是一种重要的工业化学品和燃料,在液体燃料电池中的应用引起了广泛的关注。理想情况下,肼可以通过氨的直接氧化偶联来制备,但热力学和动力学因素限制了这种方法的可行性。本研究评估了二苯甲酮亚胺(一种易于获得的氨替代物)氧化自偶联的三种不同电化学策略。所得二苯甲酮吖嗪水解得到肼和二苯甲酮,后者可回收。三种不同的电化学神经网络耦合方法包括(1)由磷酸盐碱促进的质子耦合电子转移过程,(2)涉及中间NI键形成的碘介导反应,以及(3)铜催化的神经网络耦合过程。对这些电化学亚胺到吖嗪氧化反应的热力学效率的分析表明,铜和碘介导的过程具有较低的过电势 (η)(分别为 390 和 470 mV),但质子耦合途径的过电势值要高得多(η) 〜1.6V)。类似的方法用于评估将氨电化学氧化为氮气的分子电催化方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号