当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Differences in the Mechanisms of MnO2 Oxidation between Lignin Model Compounds with the p-Hydroxyphenyl, Guaiacyl, and Syringyl Nuclei.
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-06-10 , DOI: 10.1021/acs.jafc.0c01956 Shirong Sun 1 , Takuya Akiyama 2 , Tomoya Yokoyama 1 , Yuji Matsumoto 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-06-10 , DOI: 10.1021/acs.jafc.0c01956 Shirong Sun 1 , Takuya Akiyama 2 , Tomoya Yokoyama 1 , Yuji Matsumoto 2
Affiliation
|
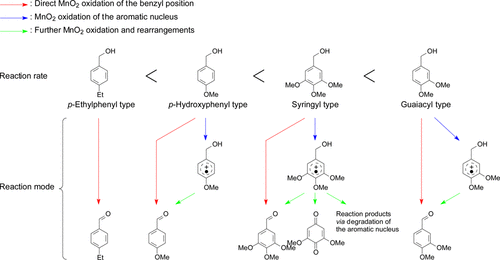
The purpose of this study was to examine how the rate and mechanism of MnO2 oxidation differ between the p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) types of simple nonphenolic lignin model compounds as well as the p-ethylphenyl (E) type compounds. The oxidation was conducted using an excess amount of MnO2 in a sulfate buffer solution at a pH value of 1.5 at room temperature. MnO2 oxidized at least the G and S nuclei, although it commonly oxidizes alcohols present at the benzyl position. The oxidation rates of the benzyl alcohol derivatives were in the order of G- > S- ≫ H- > E-type, which suggests that the rates are determined by the electronic effects of their methoxy and ethyl functional groups on not only their benzyl positions but also their aromatic π-electron systems. The kinetic isotope effect was observed in the MnO2 oxidations of the same derivatives deuterated at their benzyl hydroxymethyl groups. The observed magnitudes were in the order of E- ≫ H- > G- ≫ S-type, suggesting that the contribution of oxidation of their aromatic nuclei, which is another reaction mode of the oxidation of their benzyl positions, increases in the reverse order.
中文翻译:

具有对羟基苯基,瓜酰基和丁香基核的木质素模型化合物之间MnO2氧化机理的差异。
本研究的目的是审查的MnO的速率和机制如何2氧化之间不同p -羟基苯基(H),愈创木基(G),和紫丁香(S)类型的简单非酚类木质素模型化合物以及所述p -乙基苯基(E)型化合物。在室温下,在pH值为1.5的硫酸盐缓冲溶液中使用过量的MnO 2进行氧化。的MnO 2尽管它通常氧化存在于苄基位置的醇,但至少氧化了G和S核。苯甲醇衍生物的氧化速率为G-> S-≫ H-> E型,这表明该速率不仅取决于其苄基位置上的甲氧基和乙基官能团的电子效应而且还有它们的芳香π电子体系。在相同的苄基羟甲基氘化的衍生物的MnO 2氧化中观察到动力学同位素效应。观察到的幅度为E-≫H-> G-≫S-型,表明其芳核的氧化贡献(即其苄基位置氧化的另一种反应方式)以相反的顺序增加。
更新日期:2020-06-24
中文翻译:

具有对羟基苯基,瓜酰基和丁香基核的木质素模型化合物之间MnO2氧化机理的差异。
本研究的目的是审查的MnO的速率和机制如何2氧化之间不同p -羟基苯基(H),愈创木基(G),和紫丁香(S)类型的简单非酚类木质素模型化合物以及所述p -乙基苯基(E)型化合物。在室温下,在pH值为1.5的硫酸盐缓冲溶液中使用过量的MnO 2进行氧化。的MnO 2尽管它通常氧化存在于苄基位置的醇,但至少氧化了G和S核。苯甲醇衍生物的氧化速率为G-> S-≫ H-> E型,这表明该速率不仅取决于其苄基位置上的甲氧基和乙基官能团的电子效应而且还有它们的芳香π电子体系。在相同的苄基羟甲基氘化的衍生物的MnO 2氧化中观察到动力学同位素效应。观察到的幅度为E-≫H-> G-≫S-型,表明其芳核的氧化贡献(即其苄基位置氧化的另一种反应方式)以相反的顺序增加。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号