当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ruthenium Catalyzed Direct Asymmetric Reductive Amination of Simple Aliphatic Ketones Using Ammonium Iodide and Hydrogen
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2020-06-10 , DOI: 10.1002/ejoc.202000750
Tamal Ghosh 1 , Martin Ernst 2 , A. Stephen K. Hashmi 1, 3 , Thomas Schaub 1, 2
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2020-06-10 , DOI: 10.1002/ejoc.202000750
Tamal Ghosh 1 , Martin Ernst 2 , A. Stephen K. Hashmi 1, 3 , Thomas Schaub 1, 2
Affiliation
|
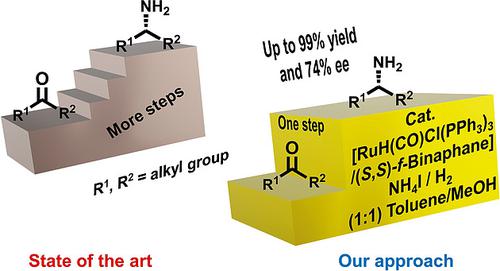
On the direct asymmetric reductive amination of aliphatic ketones to primary amines: By using Ru‐Binaphane as catalyst and NH4I as the amine source, it is possible to aminate prochiral aliphatic ketones with moderate ee values up to 74 %.

中文翻译:

碘化铵和氢催化钌催化简单脂肪族酮的不对称直接还原胺化
在脂肪族酮与伯胺的直接不对称还原胺化上:通过使用Ru-Binaphane作为催化剂和NH 4 I作为胺源,可以胺化中等ee值高达74%的前手性脂肪族酮。

更新日期:2020-08-10

中文翻译:

碘化铵和氢催化钌催化简单脂肪族酮的不对称直接还原胺化
在脂肪族酮与伯胺的直接不对称还原胺化上:通过使用Ru-Binaphane作为催化剂和NH 4 I作为胺源,可以胺化中等ee值高达74%的前手性脂肪族酮。
































 京公网安备 11010802027423号
京公网安备 11010802027423号