当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering Isolated Mn-N2C2 Atomic Interface Sites for Efficient Bifunctional Oxygen Reduction and Evolution Reaction.
Nano Letters ( IF 9.6 ) Pub Date : 2020-06-09 , DOI: 10.1021/acs.nanolett.0c01925
Huishan Shang 1 , Wenming Sun 2 , Rui Sui 3 , Jiajing Pei 3 , Lirong Zheng 4 , Juncai Dong 4 , Zhuoli Jiang 1 , Danni Zhou 1 , Zhongbin Zhuang 3 , Wenxing Chen 1 , Jiatao Zhang 1 , Dingsheng Wang 5 , Yadong Li 5
Nano Letters ( IF 9.6 ) Pub Date : 2020-06-09 , DOI: 10.1021/acs.nanolett.0c01925
Huishan Shang 1 , Wenming Sun 2 , Rui Sui 3 , Jiajing Pei 3 , Lirong Zheng 4 , Juncai Dong 4 , Zhuoli Jiang 1 , Danni Zhou 1 , Zhongbin Zhuang 3 , Wenxing Chen 1 , Jiatao Zhang 1 , Dingsheng Wang 5 , Yadong Li 5
Affiliation
|
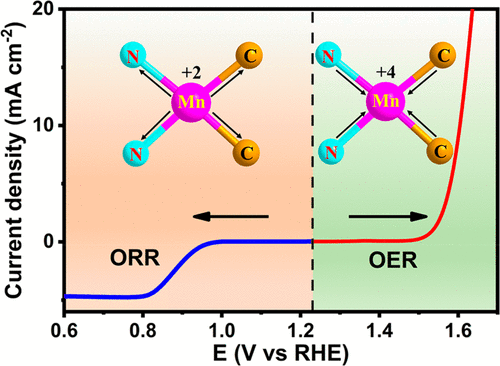
Oxygen-involved electrochemical reactions are crucial for plenty of energy conversion techniques. Herein, we rationally designed a carbon-based Mn–N2C2 bifunctional electrocatalyst. It exhibits a half-wave potential of 0.915 V versus reversible hydrogen electrode for oxygen reduction reaction (ORR), and the overpotential is 350 mV at 10 mA cm–2 during oxygen evolution reaction (OER) in alkaline condition. Furthermore, by means of operando X-ray absorption fine structure measurements, we reveal that the bond-length-extended Mn2+–N2C2 atomic interface sites act as active centers during the ORR process, while the bond-length-shortened high-valence Mn4+–N2C2 moieties serve as the catalytic sites for OER, which is consistent with the density functional theory results. The atomic and electronic synergistic effects for the isolated Mn sites and the carbon support play a critical role to promote the oxygen-involved catalytic performance, by regulating the reaction free energy of intermediate adsorption. Our results give an atomic interface strategy for nonprecious bifunctional single-atom electrocatalysts.
中文翻译:

工程分离的Mn-N2C2原子界面位点,可有效地进行双功能氧还原和放出反应。
涉及氧气的电化学反应对于大量的能量转换技术至关重要。在这里,我们合理地设计了一种碳基Mn–N 2 C 2双功能电催化剂。与可逆氢电极进行氧还原反应(ORR)相比,它的半波电势为0.915 V,在碱性条件下的氧发生反应(OER)时,在10 mA cm –2时的超电势为350 mV 。此外,通过操作X射线吸收精细结构测量,我们发现在ORR过程中,键长延伸的Mn 2+ –N 2 C 2原子界面位点充当活性中心,而键长缩短高价Mn 4+ –N2 C 2部分作为OER的催化位点,这与密度泛函理论结果一致。通过调节中间吸附的反应自由能,离析的Mn位和碳载体的原子和电子协同效应起着至关重要的作用,以促进涉及氧气的催化性能。我们的结果为非贵金属双功能单原子电催化剂提供了一种原子界面策略。
更新日期:2020-07-08
中文翻译:

工程分离的Mn-N2C2原子界面位点,可有效地进行双功能氧还原和放出反应。
涉及氧气的电化学反应对于大量的能量转换技术至关重要。在这里,我们合理地设计了一种碳基Mn–N 2 C 2双功能电催化剂。与可逆氢电极进行氧还原反应(ORR)相比,它的半波电势为0.915 V,在碱性条件下的氧发生反应(OER)时,在10 mA cm –2时的超电势为350 mV 。此外,通过操作X射线吸收精细结构测量,我们发现在ORR过程中,键长延伸的Mn 2+ –N 2 C 2原子界面位点充当活性中心,而键长缩短高价Mn 4+ –N2 C 2部分作为OER的催化位点,这与密度泛函理论结果一致。通过调节中间吸附的反应自由能,离析的Mn位和碳载体的原子和电子协同效应起着至关重要的作用,以促进涉及氧气的催化性能。我们的结果为非贵金属双功能单原子电催化剂提供了一种原子界面策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号