当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Equilibrium in the Ternary System K2O–Al2O3–H2O at 323.15, 333.15, 343.15, and 353.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.jced.0c00017
Mengjie Luo 1 , Junxiang Ye 1 , Jin Xue 1 , Chenglin Liu 1 , Xingfu Song 1 , Jianguo Yu 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.jced.0c00017
Mengjie Luo 1 , Junxiang Ye 1 , Jin Xue 1 , Chenglin Liu 1 , Xingfu Song 1 , Jianguo Yu 1
Affiliation
|
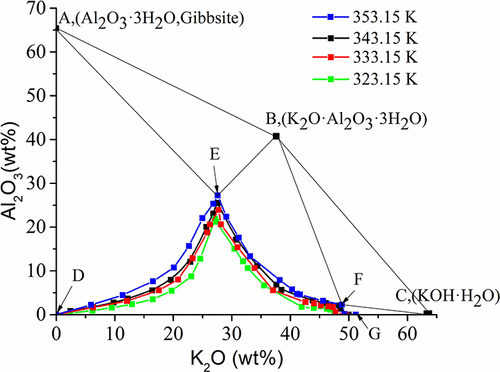
Phase equilibrium of ternary system K2O–Al2O3–H2O is the key in realizing seeded precipitation process of aluminum hydroxide product from alkaline leaching solution in the alunite tailings metallurgical industry. However, little work has been carried out about the solubility of the K2O–Al2O3–H2O system, especially for the concentrated alkali solution region. The corresponding phase equilibria at 323.15, 333.15, 343.15, and 353.15 K were researched by using the isothermal dissolution method and the Pitzer ion-interaction model. The calculated Al2O3·3H2O solubilities corresponding to the crystallization region of Al2O3·3H2O in the ternary system at each temperature basically agree with experimental values when K2O concentration is below 30 wt %. Furthermore, the ternary system at each temperature contains two invariant points, three univariant isothermal dissolution curves, and five crystallization fields corresponding to Al2O3·3H2O (gibbsite), K2O·Al2O3·3H2O, KOH·H2O, Al2O3·3H2O + K2O·Al2O3·3H2O, and K2O·Al2O3·3H2O + KOH·H2O. According to the phase diagrams of the ternary system, it can be indicated that the low alkali concentration and temperature are in favor of the crystallization of Al2O3·3H2O, while the high alkali concentration and low temperature are beneficial to the crystallization of K2O·Al2O3·3H2O.
中文翻译:

三元体系K 2 O–Al 2 O 3 –H 2 O在323.15、333.15、343.15和353.15 K时的相平衡
三元体系K 2 O–Al 2 O 3 –H 2 O的相平衡是实现轻石尾矿冶金行业碱浸液中氢氧化铝产物的晶种沉淀过程的关键。但是,关于K 2 O–Al 2 O 3 –H 2 O系统的溶解度,尤其是在浓碱溶液区域,几乎没有进行任何工作。利用等温溶解方法和Pitzer离子相互作用模型研究了323.15、333.15、343.15和353.15 K处的相应相平衡。计算出的Al 2 O 3 ·3H 2当K 2 O浓度低于30wt%时,在每个温度下与三元体系中Al 2 O 3 ·3H 2 O的结晶区域相对应的O溶解度基本上与实验值一致。此外,每个温度下的三元体系包含两个不变点,三个单变量等温溶解曲线和五个结晶场,分别对应于Al 2 O 3 ·3H 2 O(三水铝石),K 2 O·Al 2 O 3 ·3H 2 O, KOH·H 2 O,Al 2 O 3 ·3H 2 O + K 2O·铝2 ö 3 ·3H 2 O和K 2 O·铝2 ö 3 ·3H 2 O + KOH·H 2 O.根据三元体系的相图,可以表明,低碱浓度温度和温度有利于Al 2 O 3 ·3H 2 O的结晶,而高碱浓度和低温有利于K 2 O·Al 2 O 3 ·3H 2 O的结晶。
更新日期:2020-07-09
中文翻译:

三元体系K 2 O–Al 2 O 3 –H 2 O在323.15、333.15、343.15和353.15 K时的相平衡
三元体系K 2 O–Al 2 O 3 –H 2 O的相平衡是实现轻石尾矿冶金行业碱浸液中氢氧化铝产物的晶种沉淀过程的关键。但是,关于K 2 O–Al 2 O 3 –H 2 O系统的溶解度,尤其是在浓碱溶液区域,几乎没有进行任何工作。利用等温溶解方法和Pitzer离子相互作用模型研究了323.15、333.15、343.15和353.15 K处的相应相平衡。计算出的Al 2 O 3 ·3H 2当K 2 O浓度低于30wt%时,在每个温度下与三元体系中Al 2 O 3 ·3H 2 O的结晶区域相对应的O溶解度基本上与实验值一致。此外,每个温度下的三元体系包含两个不变点,三个单变量等温溶解曲线和五个结晶场,分别对应于Al 2 O 3 ·3H 2 O(三水铝石),K 2 O·Al 2 O 3 ·3H 2 O, KOH·H 2 O,Al 2 O 3 ·3H 2 O + K 2O·铝2 ö 3 ·3H 2 O和K 2 O·铝2 ö 3 ·3H 2 O + KOH·H 2 O.根据三元体系的相图,可以表明,低碱浓度温度和温度有利于Al 2 O 3 ·3H 2 O的结晶,而高碱浓度和低温有利于K 2 O·Al 2 O 3 ·3H 2 O的结晶。

































 京公网安备 11010802027423号
京公网安备 11010802027423号