当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spectroscopic Investigation of Cysteamine Dioxygenase.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.biochem.0c00267 Rebeca L Fernandez 1 , Stephanie L Dillon 1 , Martha H Stipanuk 2 , Brian G Fox 3 , Thomas C Brunold 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-08 , DOI: 10.1021/acs.biochem.0c00267 Rebeca L Fernandez 1 , Stephanie L Dillon 1 , Martha H Stipanuk 2 , Brian G Fox 3 , Thomas C Brunold 1
Affiliation
|
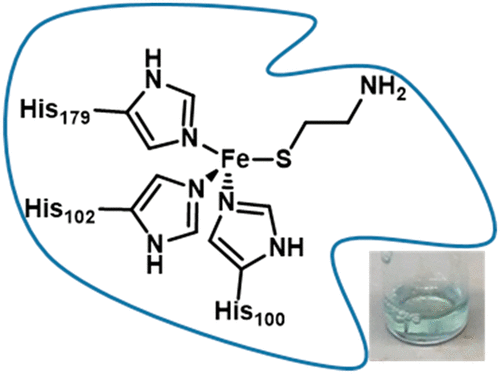
Thiol dioxygenases are mononuclear non-heme FeII-dependent metalloenzymes that initiate the oxidative catabolism of thiol-containing substrates to their respective sulfinates. Cysteine dioxygenase (CDO), the best characterized mammalian thiol dioxygenase, contains a three-histidine (3-His) coordination environment rather than the 2-His-1-carboxylate facial triad seen in most mononuclear non-heme FeII enzymes. A similar 3-His active site is found in the bacterial thiol dioxygenase 3-mercaptopropionate dioxygenase (MDO), which converts 3-mercaptopropionate into 3-sulfinopropionic acid as part of the bacterial sulfur metabolism pathway. In this study, we have investigated the active site geometric and electronic structures of a third non-heme FeII-dependent thiol dioxygenase, cysteamine dioxygenase (ADO), by using a spectroscopic approach. Although a 3-His facial triad had previously been implicated on the basis of sequence alignment and site-directed mutagenesis studies, little is currently known about the active site environment of ADO. Our magnetic circular dichroism and electron paramagnetic resonance data provide compelling evidence that ADO features a 3-His facial triad, like CDO and MDO. Despite this similar coordination environment, spectroscopic results obtained for ADO incubated with various substrate analogues are distinct from those obtained for the other FeII-dependent thiol dioxygenases. This finding suggests that the secondary coordination sphere of ADO is distinct from those of CDO and MDO, demonstrating the significant role that secondary-sphere residues play in dictating substrate specificity.
中文翻译:

半胱胺双加氧酶的光谱研究。
硫醇双加氧酶是单核非血红素依赖Fe II的金属酶,可引发含硫醇的底物向其各自的亚磺酸盐的氧化分解代谢。半胱氨酸双加氧酶(CDO)是最有特色的哺乳动物巯基双加氧酶,它包含一个三组氨酸(3-His)配位环境,而不是大多数单核非血红素Fe II酶中的2-His-1-羧化物面部三联体。在细菌硫醇双加氧酶3-巯基丙酸酯双加氧酶(MDO)中发现了类似的3-His活性位点,该酶将3-巯基丙酸酯转化为3-磺基丙酸,作为细菌硫代谢途径的一部分。在这项研究中,我们研究了第三种非血红素Fe II的活性位几何和电子结构依赖的巯基双加氧酶,半胱胺双加氧酶(ADO),通过使用光谱方法。尽管以前曾根据序列比对和定点诱变研究牵涉到3-His面部三联征,但目前对ADO的活性位点环境知之甚少。我们的磁性圆二色性和顺磁共振电子数据提供了令人信服的证据,证明ADO具有3-His面部三联征,如CDO和MDO。尽管有类似的配位环境,但使用各种底物类似物孵育的ADO所获得的光谱结果与其他Fe II所获得的光谱结果却截然不同依赖性硫醇双加氧酶。这一发现表明,ADO的二级配位区与CDO和MDO的二级配位区不同,这表明二级配位区残基在决定底物特异性方面起着重要作用。
更新日期:2020-07-07
中文翻译:

半胱胺双加氧酶的光谱研究。
硫醇双加氧酶是单核非血红素依赖Fe II的金属酶,可引发含硫醇的底物向其各自的亚磺酸盐的氧化分解代谢。半胱氨酸双加氧酶(CDO)是最有特色的哺乳动物巯基双加氧酶,它包含一个三组氨酸(3-His)配位环境,而不是大多数单核非血红素Fe II酶中的2-His-1-羧化物面部三联体。在细菌硫醇双加氧酶3-巯基丙酸酯双加氧酶(MDO)中发现了类似的3-His活性位点,该酶将3-巯基丙酸酯转化为3-磺基丙酸,作为细菌硫代谢途径的一部分。在这项研究中,我们研究了第三种非血红素Fe II的活性位几何和电子结构依赖的巯基双加氧酶,半胱胺双加氧酶(ADO),通过使用光谱方法。尽管以前曾根据序列比对和定点诱变研究牵涉到3-His面部三联征,但目前对ADO的活性位点环境知之甚少。我们的磁性圆二色性和顺磁共振电子数据提供了令人信服的证据,证明ADO具有3-His面部三联征,如CDO和MDO。尽管有类似的配位环境,但使用各种底物类似物孵育的ADO所获得的光谱结果与其他Fe II所获得的光谱结果却截然不同依赖性硫醇双加氧酶。这一发现表明,ADO的二级配位区与CDO和MDO的二级配位区不同,这表明二级配位区残基在决定底物特异性方面起着重要作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号