当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carboxylate-Assisted β-(Z) Stereoselective Hydrosilylation of Terminal Alkynes Catalyzed by a Zwitterionic Bis-NHC Rhodium(III) Complex
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-05 , DOI: 10.1021/acscatal.0c01582 Raquel Puerta-Oteo 1 , Julen Munarriz 2, 3 , Víctor Polo 2 , M. Victoria Jiménez 1 , Jesús J. Pérez-Torrente 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-05 , DOI: 10.1021/acscatal.0c01582 Raquel Puerta-Oteo 1 , Julen Munarriz 2, 3 , Víctor Polo 2 , M. Victoria Jiménez 1 , Jesús J. Pérez-Torrente 1
Affiliation
|
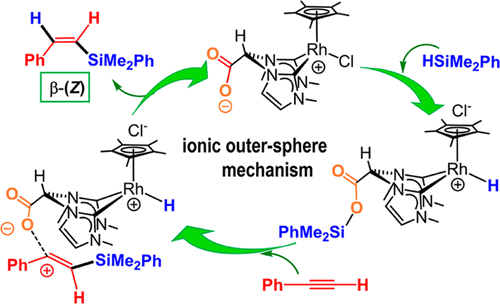
The zwitterionic compound [Cp*RhCl{(MeIm)2CHCOO}] is an efficient catalyst for the hydrosilylation of terminal alkynes with excellent regio- and stereoselectivity toward the less thermodynamically stable β-(Z)-vinylsilane isomer under mild reaction conditions. A broad range of linear 1-alkynes, cycloalkyl acetylenes, and aromatic alkynes undergo the hydrosilylation with HSiMe2Ph to afford the corresponding β-(Z)-vinylsilanes in quantitative yields in short reaction times. The reaction of aliphatic alkynes with HSiEt3 is slower, resulting in a slight decrease of selectivity toward the β-(Z)-vinylsilane product, which is still greater than 90%. However, a significant selectivity decrease is observed in the hydrosilylation of aromatic alkynes because of the β-(Z) → β-(E) vinylsilane isomerization. Moreover, the hydrosilylation of bulky alkynes, such as t-Bu–C≡CH or Et3SiC≡CH, is unselective. Experimental evidence suggests that the carboxylate function plays a key role in the reaction mechanism, which has been validated by means of density functional theory calculations, as well as by mass spectrometry and labeling studies. On the basis of previous results, we propose an ionic outer-sphere mechanism pathway in which the carboxylate fragment acts as a silyl carrier. Namely, the hydrosilylation mechanism entails the heterolytic activation of the hydrosilane assisted by the carboxylate function to give the hydrido intermediate [Cp*RhH{(MeIm)2CHCOO–SiR3}]+. The transference of the silylium moiety from the carboxylate to the alkyne results in the formation of a flat β-silyl carbocation intermediate that undergoes a hydride transfer from the Rh(III) center to generate the vinylsilane product. The outstanding β-(Z) selectivity results from the minimization of the steric interaction between the silyl moiety and the ligand system in the hydride transfer transition state.
中文翻译:

两性离子双-NHC铑(III)配合物催化末端炔烃的羧酸盐辅助β-(Z)立体选择性氢化硅烷化
两性离子化合物[Cp * RhCl {(MeIm)2 CHCOO}]是一种有效的催化剂,可在温和的反应条件下,使末端炔烃对热力学上较不稳定的β-(Z)-乙烯基硅烷异构体具有出色的区域选择性和立体选择性。各种各样的线性1-炔烃,环烷基乙炔和芳族炔烃与HSiMe 2 Ph进行氢化硅烷化反应,可以在较短的反应时间内以定量收率得到相应的β-(Z)-乙烯基硅烷。脂族炔烃与HSiEt 3的反应较慢,导致对β-(Z的选择性略微降低)-乙烯基硅烷产物,其仍大于90%。然而,由于β-(Z)→β-(E)乙烯基硅烷异构化,在芳族炔烃的氢化硅烷化中观察到显着的选择性降低。此外,大体积炔烃(如t -Bu–C≡CH或Et 3)的氢化硅烷化SiC≡CH是非选择性的。实验证据表明,羧酸盐功能在反应机理中起着关键作用,已通过密度泛函理论计算以及质谱和标记研究进行了验证。根据先前的结果,我们提出了一种离子外球机理途径,其中羧酸盐片段充当甲硅烷基载体。也就是说,氢化硅烷化机理需要通过羧酸酯功能辅助氢化硅烷的杂化活化,从而得到氢化中间体[Cp * RhH {(MeIm)2 CHCOO–SiR 3 }] +。甲硅烷基部分从羧酸酯到炔的转移导致形成平坦的β-甲硅烷基碳阳离子中间体,该中间体经历从Rh(III)中心的氢化物转移以生成乙烯基硅烷产物。出色的β-(Z)选择性是由于在氢化物转移过渡态中,甲硅烷基部分与配体系统之间的空间相互作用最小而产生的。
更新日期:2020-07-02
中文翻译:

两性离子双-NHC铑(III)配合物催化末端炔烃的羧酸盐辅助β-(Z)立体选择性氢化硅烷化
两性离子化合物[Cp * RhCl {(MeIm)2 CHCOO}]是一种有效的催化剂,可在温和的反应条件下,使末端炔烃对热力学上较不稳定的β-(Z)-乙烯基硅烷异构体具有出色的区域选择性和立体选择性。各种各样的线性1-炔烃,环烷基乙炔和芳族炔烃与HSiMe 2 Ph进行氢化硅烷化反应,可以在较短的反应时间内以定量收率得到相应的β-(Z)-乙烯基硅烷。脂族炔烃与HSiEt 3的反应较慢,导致对β-(Z的选择性略微降低)-乙烯基硅烷产物,其仍大于90%。然而,由于β-(Z)→β-(E)乙烯基硅烷异构化,在芳族炔烃的氢化硅烷化中观察到显着的选择性降低。此外,大体积炔烃(如t -Bu–C≡CH或Et 3)的氢化硅烷化SiC≡CH是非选择性的。实验证据表明,羧酸盐功能在反应机理中起着关键作用,已通过密度泛函理论计算以及质谱和标记研究进行了验证。根据先前的结果,我们提出了一种离子外球机理途径,其中羧酸盐片段充当甲硅烷基载体。也就是说,氢化硅烷化机理需要通过羧酸酯功能辅助氢化硅烷的杂化活化,从而得到氢化中间体[Cp * RhH {(MeIm)2 CHCOO–SiR 3 }] +。甲硅烷基部分从羧酸酯到炔的转移导致形成平坦的β-甲硅烷基碳阳离子中间体,该中间体经历从Rh(III)中心的氢化物转移以生成乙烯基硅烷产物。出色的β-(Z)选择性是由于在氢化物转移过渡态中,甲硅烷基部分与配体系统之间的空间相互作用最小而产生的。































 京公网安备 11010802027423号
京公网安备 11010802027423号