当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Zinc/Indium Bimetallic Lewis Acid Relay Catalysis for Dehydrogenative Silylation/Hydrosilylation Reaction of Terminal Alkynes with Bis(hydrosilane)s
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-07 , DOI: 10.1002/adsc.202000501 Tomohiro Tani 1 , Yudai Sohma 1 , Teruhisa Tsuchimoto 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-06-07 , DOI: 10.1002/adsc.202000501 Tomohiro Tani 1 , Yudai Sohma 1 , Teruhisa Tsuchimoto 1
Affiliation
|
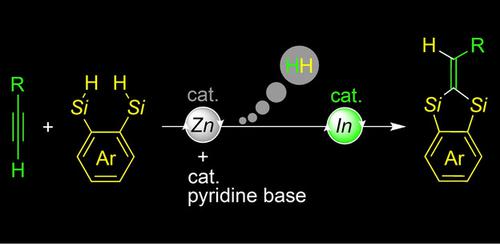
When mixed with two different Lewis acid catalysts of zinc and indium, terminal alkynes were found to react with bis(hydrosilane)s to selectively provide 1,1‐disilylalkenes from among several possible products, by way of a sequential dehydrogenative silylation/intramolecular hydrosilylation reaction. Adding a pyridine base is crucial in this reaction; a switch as a catalyst of the zinc Lewis acid is turned on by forming a zinc−pyridine‐base complex. A range of the 1,1‐disilylalkenes can be obtained by a combination of aryl and aliphatic terminal alkynes plus aryl‐, heteroaryl‐, and naphthyl‐tethered bis(hydrosilane)s. The 1,1‐disilylalkene prepared here is available as a reagent for further transformations by utilizing its C−Si or C=C bond. The former includes Hiyama cross‐coupling, bismuth‐catalyzed ether formation, and iododesilylation; the latter includes double alkylation and epoxidation. Mechanistic studies clarified the role of the two Lewis acids: the zinc–pyridine‐base complex catalyzes the dehydrogenative silylation as a first stage, and, following on this, the indium Lewis acid catalyzes the ring‐closing hydrosilylation as a second stage, thus leading to the 1,1‐disilylalkene.
中文翻译:

锌/铟双金属路易斯酸中继催化剂催化末端炔烃与双(氢硅烷)的脱氢硅烷化/氢化硅烷化反应
当与两种不同的锌和铟的路易斯酸催化剂混合时,发现末端炔烃与双(氢硅烷)反应,通过顺序脱氢甲硅烷基化/分子内氢化硅烷化反应,从几种可能的产物中选择性地提供1,1-二甲硅烷基烯烃。 。在该反应中添加吡啶碱至关重要。通过形成锌吡啶基络合物来打开作为锌路易斯酸催化剂的开关。可以通过芳基和脂族末端炔烃与芳基,杂芳基和萘系双(氢硅烷)的组合获得一系列1,1-二甲硅烷基烯烃。通过使用其C-Si或C = C键,此处制备的1,1-二甲硅烷基烯烃可作进一步转化的试剂。前者包括Hiyama交叉偶联,铋催化的醚形成和碘去甲硅烷基化。后者包括双烷基化和环氧化。机理研究阐明了这两种路易斯酸的作用:锌-吡啶基络合物作为第一步催化脱氢甲硅烷基化,随后,铟路易斯酸作为第二步催化闭环氢化硅烷化,从而导致1,1-二甲硅烷基。
更新日期:2020-06-07
中文翻译:

锌/铟双金属路易斯酸中继催化剂催化末端炔烃与双(氢硅烷)的脱氢硅烷化/氢化硅烷化反应
当与两种不同的锌和铟的路易斯酸催化剂混合时,发现末端炔烃与双(氢硅烷)反应,通过顺序脱氢甲硅烷基化/分子内氢化硅烷化反应,从几种可能的产物中选择性地提供1,1-二甲硅烷基烯烃。 。在该反应中添加吡啶碱至关重要。通过形成锌吡啶基络合物来打开作为锌路易斯酸催化剂的开关。可以通过芳基和脂族末端炔烃与芳基,杂芳基和萘系双(氢硅烷)的组合获得一系列1,1-二甲硅烷基烯烃。通过使用其C-Si或C = C键,此处制备的1,1-二甲硅烷基烯烃可作进一步转化的试剂。前者包括Hiyama交叉偶联,铋催化的醚形成和碘去甲硅烷基化。后者包括双烷基化和环氧化。机理研究阐明了这两种路易斯酸的作用:锌-吡啶基络合物作为第一步催化脱氢甲硅烷基化,随后,铟路易斯酸作为第二步催化闭环氢化硅烷化,从而导致1,1-二甲硅烷基。































 京公网安备 11010802027423号
京公网安备 11010802027423号